Abstract
Aim. To investigate antiproteinuric effect of spironolactone in patients with chronic kidney disease (CKD) treated with angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin II type 1 receptor blockers (ARBs). Methods. This study was performed in 33 CKD patients with proteinuria. 24 h urinary protein excretion and biochemical parameters were obtained before the therapy. Then, spironolactone (25 mg/d) was added to the therapy. The antiproteinuric effect of spironolactone was examined for eight weeks. Results. At eight weeks, there was a significant decrease in proteinuria (p < 0.001, 47.9% decrease). Systolic and diastolic blood pressures were significantly decreased (p < 0.004, p < 0.001, respectively). However, no correlation was detected between the reductions in systolic and diastolic BP and the reduction in proteinuria (p = 0.464, p = 0.239, respectively). Serum potassium level increased significantly (p < 0.001). Conclusions. Our study suggests that spironolactone significantly reduces urinary protein excretion. This strategy may be useful to slow the progression of CKD. However, hyperkalemia is the most important side effect of treatment, and it is necessary to monitor potassium level. Further studies are needed to determine the efficacy of spironolactone on proteinuria.
INTRODUCTION
Chronic kidney disease (CKD) is a major public health issue. The importance of renin-angiotensin-aldosterone system (RAAS) in the progression of CKD has been clearly demonstrated. RAAS blockade with angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin-receptor blockers (ARBs) has become a leading therapeutic strategy to slow the progression of CKD.Citation[1–3]
The level of urinary protein excretion is one of the most important factors affecting renal outcomes. The higher the urinary protein excretion, the faster the decline in glomerular filtration rate (GFR). The reduction in proteinuria is also established to slow the progressive reduction in GFR. ACE inhibitors and ARBs are known to decrease proteinuria.Citation[2,Citation4] Although these drugs are effective, most patients progress to end stage renal disease.
Aldosterone, the end product of RAAS, is a major mediator of renal disease. It has been revealed that aldosterone directly contributes to renal and cardiovascular diseases by stimulating endothelial dysfunction, inflammation, and fibrosis.Citation[5,Citation6]
Although plasma aldosterone level is suppressed at the beginning of treatment with ACE inhibitors or ARBs, subsequently, aldosterone level may increase to pretreatment levels. This phenomenon is known as the aldosterone escape. Also, it has been shown that urinary protein excretion was higher and decrease in GFR was faster in patients with escape.Citation[7,Citation8]
Spironolactone is an agent that directly suppresses the effect of aldosterone.Citation[9] Data on the ability of spironolactone to decrease proteinuria or affect progression of renal disease are inadequate.
This study is designed to investigate whether aldosterone antagonism by spironolactone has any effect on urinary protein excretion in patients with CKD.
PATIENTS AND METHODS
Study Design and Patients
This is a prospective, uncontrolled study investigating effect of spironolactone on urinary protein excretion. The study was performed in 33 patients (17 males and 16 females) who have overt proteinuria (> 300 mg/day) despite regular use of ACE inhibitors and/or ARBs for at least six months. The mean age was 45.21 ± 14.37 years, and the body mass index was 28.59 ± 7.06 kg/m2. The etiology of CKD was diabetes mellitus in 20 patients (60.6%), chronic glomerulonephritis in 10 patients (30.3%), and amyloidosis in three patients (9.1%). Eight (40%), seven (35%), and three (15%) patients with diabetes mellitus were using insulin, oral antidiabetic drugs (OAD), and insulin in combination with OAD, respectively. Two patients (10%) were not using any medication, and blood glucose was managed by diet. 20 (60.6%), 11 (33.3%), and 2 (6.1%) of the cases were using ACE inhibitors, ARBs, and ACE inhibitors combined with ARBs, respectively. After the initial evaluation and laboratory measurements, 25 mg of spironolactone was added to the antihypertensive medication of patients. Criteria for exclusion were stage 4 and stage 5 CKD (creatinine clearance below 30 mL/min), serum potassium level above 5 mEq/L at study initiation or prior to study, congestive heart failure, chronic liver disease, malignancy, use of corticosteroids, or immunosuppressive drugs.
Patients who meet the inclusion criteria were informed of the study, and written consent was obtained. The study protocol was approved by the Ethical Committee of Kocaeli University Medical Faculty.
Laboratory
All biochemical parameters and urinary analysis were performed at the Biochemistry Laboratory of Kocaeli University Medical Faculty. Patients were informed on 24-hour urine collection. Venous blood samples were obtained after 8–12 hours of fasting, in the morning in a sitting position.
Blood pressure (BP) was measured by a cuff-type sphygmomanometer device. After at least 10 minutes of sitting, the mean of three BP measurements obtained from the right arm at 10-minute intervals was calculated. Korotkoff's first sound was recorded as systolic BP and Korotkoff's fifth sound was recorded as diastolic BP.
At one week of the treatment, venous blood sample was taken to control serum potassium level. Patients were also seen at four and after weeks of the treatment. At each control, biochemical and urinary analysis were repeated, the mean of the three BP measurements obtained from the right arm was recorded, and patients were interrogated with respect to side effects such as potentially treatment-related gastrointestinal intolerance and gynecomastia.
Blood and urine samples were analyzed on a Hitachi Modular P800 (Roche Diagnostics, Japan) following the manufacturer's recommendations. Urinary protein excretion was measured with turbidimetric method. Serum creatinine level was measured with alkaline picrate method. Serum potassium level was determined with ion selective electrode method. The BUN level was determined with urease method. Serum albumin level was measured by binding of albumin and bromcresol green for formation of a colored complex. Creatinine clearance was calculated according to the following formula:
where UC = urinary creatinine level (mg/dL), UV = urine volume (mL/d), SC = serum creatinine level (mg/dL), and 1440 = value of the 24-hour period (in minutes).
Statistical Analysis
The statistical analysis was performed using SPSS version 13.0. Data are expressed as mean ± standard deviation. The characteristics of distribution were tested using the Kolmogorov– Smirnov test. The difference in proteinuria level between pre-treatment and at four weeks was evaluated by Wilcoxon signed ranks test. Repeated measures analysis of variance was used for comparison of changes in parameters over time. Serum potassium and albumin levels were compared by paired-samples test. Data except for creatinine between normokalemic and hyperkalemic patients were analyzed by the independent sample t test. However, Mann-Whitney U test was used for examining serum creatinine due to the absence of a normal distribution. BP was analyzed by Wilcoxon signed ranks test. p < 0.05 was considered statistically significant.
RESULTS
At four weeks of treatment, a reduction of 25.4% in proteinuria was established (p = 0.003). Serum potassium level increased a mean of 0.28 ± 0.08 mEq/L (p < 0.001). Both systolic and diastolic BP were significantly reduced (p = 0.013, p = 0.040, respectively). However, no significant correlation was detected between the reductions in systolic and diastolic BP and the reduction in proteinuria (p = 0.093, p = 0.396, respectively).
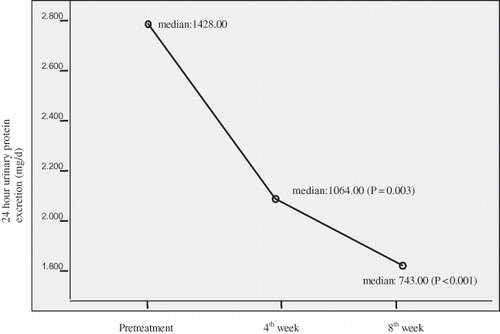
At eight weeks of therapy, the 24-hour median urinary protein excretion significantly decreased from 1428 mg/d to 743 mg/d (47.9%). The course of urinary protein excretion throughout treatment is presented in . Systolic and diastolic BP were significantly reduced. However, no correlation was detected between the reductions in systolic and diastolic BP and the reduction in proteinuria (p = 0.464, p = 0.239, respectively). A mean increase of 0.55 ± 0.32 mEq/L was detected in serum potassium level (p < 0.001). The differences in creatinine clearance and serum creatinine levels were not significant. Serum albumin level increased from 3.88 ± 0.5 g/dL to 4.01 ± 0.44 g/dL (p = 0.003). All results are presented in .
Table 1 The laboratory results, blood pressure values obtained prior to treatment and at four and eight weeks of treatment, and the relationship between them
Hyperkalemia above 5.5 mEq/L was detected in six patients (18.2%) at eight weeks of therapy. The etiology of CKD was diabetes mellitus in all of them. The baseline characteristics of the patients with and without hyperkalemia are presented in .
Table 2 The baseline characteristics of the patients with and without hyperkalemia
DISCUSSION
ACE inhibitors and ARBs are well known to decrease urinary protein excretion and slow the progression of CKD.Citation[1–4] Studies demonstrated that antiproteinuric effect of the combination of ACE inhibitors and ARBs was more marked than that of ACE inhibitors or ARBs alone.Citation[10,Citation11] However, ACE inhibitors and/or ARBs cannot eliminate CKD or decrease proteinuria to normal levels.
This condition may be explained by the incomplete blockade of the RAAS. It was found that plasma aldosterone level was increased in 40–41% of patients during the long-term treatment with ACE inhibitor or ARB. It was also observed that urinary protein excretion was higher, and there was an increased reduction in GFR in patients with aldosterone escape.Citation[7,Citation8] These results indicate that aldosterone have some important effects on protein excretion and CKD progression.
Bianchia et al.Citation[12] evaluated the effect of spironolactone on proteinuria in patients receiving ACE inhibitor and/or ARB and detected that protein excretion decreased from 2.09 ± 0.16 g/d to 1.05 ± 0.08 g/d, a 44.4% decrease, after eight weeks (p < 0.01). The assessment performed at four weeks after the study demonstrated that proteinuria increased and approached baseline levels (p < 0.05). In addition, pre-treatment proteinuria and aldosterone level and the reduction in proteinuria and the aldosterone level were detected to be significantly correlated (p < 0.0001, p < 0.0001). In the study, a significant increase was detected in serum potassium level (p < 0.01).
Sato et al. investigated the effect of spironolactone in CKD patients with proteinuria above 0.5 g/d despite long-term use of ACE inhibitor and observed that proteinuria decreased from 1162 ± 77 mg/d to 722 ± 58 mg/d (37.8% decrease) (p < 0.05).Citation[13]
In our study, the addition of spironolactone to treatment in CKD patients with overt proteinuria decreased proteinuria significantly.
In the study investigating the effect of spironolactone on urinary protein creatinine ratio (UPCR) and urinary albumin creatinine ratio (UACR) in patients with diabetic nephropathy, it has been established that the reduction in UPCR and UACR was 40.6% and 44.2%. The reduction in proteinuria and albuminuria did not correlate with baseline BP. Hyperkalemia was observed to develop in 17.2% of patients.Citation[14]
In another trial, the effect of 25 mg spironolactone on albuminuria, BP, and GFR was investigated in patients with diabetic nephropathy, and a 33% reduction was detected in albuminuria (p < 0.001) and a 6 mmHg and 4 mmHg decrease was observed in systolic and diastolic BP (p < 0.001, p < 0.001, respectively). However, no statistically significant correlation was detected between the reduction in BP and albuminuria. Hyperkalemia developed only in one patient.Citation[11]
Although some studies demonstrated that spironolactone did not decrease BP significantly,Citation[12,Citation13] some studies also detected significant reductions in BP.Citation[11,Citation14] However, no statistically significant correlation was established between proteinuria and BP reduction in these studies.
We determined that there was a statistically significant reduction in systolic and diastolic BP after treatment. However, there was no statistically significant correlation between the reduction in BP and the reduction in proteinuria. These findings have indicated that the antiproteinuric effect of spironolactone does not depend on change in BP.
Aldosterone stimulates the production of type IV collagen resulting in renal fibrosisCitation[15] and spironolactone decreases urinary type IV collagen excretion.Citation[13] As a result, the beneficial effects of spironolactone may be partially explained by the decrease in extracellular collagen cycle.
In a study performed in patients with diabetic nephropathy, the annual reduction rate in GFR was twofold higher in patients with aldosterone escape phenomenon (5 mL/min/1.73 m2) during RAS blockage than those without escape phenomenon (2.4 mL/min/1.73 m2). These results show the benefit effect of long-term aldosterone antagonism.Citation[8] Our study detected no significant change in creatinine clearance (p > 0.05).
The important side effect of combined use of the RAS inhibitors and mineralocorticoid receptor blockers is the development of hyperkalemia. After the RALES and EPHESUS studies,Citation[16,Citation17] it has been reported that spironolactone use, hospitalization, and death rates due to associated hyperkalemia rapidly increased in patients with heart failure.Citation[18] It has been also observed that hyperkalemia could result from poor potassium monitoring, careless monitoring of renal functions, high dose of spironolactone, administration of potassium and excessive potassium intake, concomitant use of drugs increasing potassium level, advanced age, type 2 diabetes mellitus, lower left ventricular ejection fraction, and higher New York Heart Association functional classification.Citation[18–20]]
In our study, a significant increase in serum potassium level was observed during the treatment and serum potassium level was above 5.5 mEq/L in six patients (18.2%). All of those patients had diabetes mellitus. The mean age (p = 0.003) and pre-treatment serum potassium levels of these patients (p = 0.006) were significantly higher than those of patients without hyperkalemia.
Although there are some important results, there are several limitations in our study. Sample size was relatively small (n = 33). The duration of study was short, and we did not have a control group.
In conclusion, our study shows that spironolactone markedly decreases proteinuria in patients with CKD, and indicates the requirement to monitor serum potassium level during spironolactone use, particularly in patients with diabetes, advanced age, high creatinine level, and high pre-treatment potassium level. Long-term comparative trials are required to demonstrate the long-term effects of aldosterone antagonism by spironolactone on CKD progression and proteinuria.
ACKNOWLEDGMENTS
The authors declare that there was no support or funding received to carry out the study, nor any other commercial relationship relevant to the article's subject matter.
REFERENCES
- Brenner BM, Cooper ME, de Zeeuw D, Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869.
- Lewis EJ, Hunsicker LG, Clarke WR, Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860.
- Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: A randomized double-blind crossover trial. Diabetes Care. 2003;26:2268–2274.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863.
- Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9.
- Brown NJ. Aldosteron and end-organ damage. Curr Opin Nephrol Hypertens. 2005;14:235–241.
- Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–68.
- Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–1939.
- Delyani JA. Mineralocorticoid receptor antagonists. The evolution of utility and pharmacology. Kidney Int. 2000;57:1408–1411.
- Jacobsen P, Andersen S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol. 2003;14:992–999.
- Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effect of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy. Diabetes Care. 2005;28:2106–2112.
- Bianchi S, Bigazzi R, Campese VM. Antagonists of aldosterone and proteinuria in patients with CKD: An uncontrolled pilot study. Am J Kidney Dis. 2005;46:45–51.
- Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005;18:44–49.
- Van den Meiracker AH, Baggen RG, Pauli S, Spironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24:2285–2292.
- Wakisaka M, Spiro MJ, Spiro RG. Synthesis of type VI collagen by cultured glomerular cells and comparison of its regulation by glucose and other factors with that of type IV collagen. Diabetes. 1994;43:95–103.
- Pitt B, Zannad F, Remme WJ, The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717.
- Pitt B, Remme W, Zannad F, Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321.
- Juurlink DN, Mamdani MM, Lee DS, Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551.
- Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. Hyperkalemia and impaired renal function in patients taking spironolactone for congestive heart failure: Retrospective study. BMJ. 2003;327:1141–1142.
- Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: An analysis of 25 cases. Am J Med. 2001;110:438–441.