Abstract
Background. Both traditional and non-traditional risk factors play a role for the development of cardiovascular disease in hemodialysis patients. However, a specific relationship between these risk factors and silent myocardial damage is unknown. Methods. Demographic, anthropometric, clinical, and laboratory data were collected. Silent myocardial damage was defined by elevated cardiac troponin I values above cutoff values. Results. In total, 113 hemodialysis patients were included. Cardiac troponin I concentrations were below cutoff value (<2.3 ng/mL) in 103 (91.2%) patients (Group 1), whereas 10 (8.8%) patients had elevated concentrations (Group 2). Group 1 patients had higher levels of hemoglobin (p = 0.002) and high-density lipoprotein cholesterol (p = 0.002) and lower C-reactive protein (p = 0.003) and tumor necrosis factor-α (p = 0.005) levels, as well as less incidence of left ventricular hypertrophy (p = 0.045), when compared to Group 2 patients. Diabetes mellitus (Beta = +0.160, p = 0.021), left ventricular hypertrophy (Beta = +0.247, p < 0.0001), uncontrolled blood pressure (Beta = +0.170, p = 0.016), normalized protein equivalent of total nitrogen appearance (Beta = −0.230, p = 0.001), hemoglobin (Beta = −0.302, p < 0.0001), and tumor necrosis factor-α (Beta = +0.506, p < 0.0001) were found to be independently associated with cardiac troponin I levels in multiple linear regression analysis. Conclusions. Both traditional and non-traditional risk factors are related with silent myocardial damage, which is considered to an antecedent of major cardiovascular events. Hemodialysis patients, even when asymptomatic, must be closely followed up for the presence of these risk factors.
INTRODUCTION
The incidence of cardiovascular death is 5–10 times greater in uremic patients than in the age-matched general population, and cardiac death is related to both traditional risk factors (e.g., advanced age, smoking, diabetes mellitus, hypertension, and dyslipidemia) as well as non-traditional ones (e.g., inflammation, malnutrition, oxidative stress, vascular calcification, and homocysteine).Citation[1,Citation2] Diagnosing cardiac disease in end-stage renal disease (ESRD) patients is difficult, because many of these patients present with abnormal baseline electrocardiograms, frequently compounded by silent or atypical symptoms, and reduced reliability of cardiac enzymes, including cardiac troponin T (cTnT).Citation[3] Cardiac troponin I (cTnI) is exclusively of cardiac origin, and unlike cTnT, is not expressed in skeletal muscle. It is suggested that cTnI is a sensitive marker of myocardial injury and is more specific for the detection of myocardial injury in ESRD patients than other cardiac enzymes.Citation[3–5] Additionally, among the troponins, especially cTnI values are found to be clearly associated with silent/subtle myocardial damage.Citation[6] Although there is considerable controversy regarding the significance of elevated cTnI levels in asymptomatic hemodialysis patients, there are studies showing that increased cTnI is a risk factor for future cardiac events, including myocardial infarction, cardiac death and revascularization.Citation[7–10] Interestingly, higher albeit normal troponin concentrations are considered to be markers of active but asymptomatic coronary artery disease, which is likely to progress to an acute coronary syndrome in relatively near future.Citation[10] Thus, a high level of troponin may not only be observed due to clinically manifest cardiovascular disease, but it might also be seen in silent/subtle myocardial damage caused by microvascular lesions or direct injury to myocardial cells (e.g., toxic, stretching, hypoxia, apoptosis). Indeed, silent/subtle myocardial damage might be considered as a precursor of more severe and clinically detectable cardiac events.Citation[11]
In this study, we analyzed the potential risk factors (both traditional and non-traditional) for silent/subtle myocardial damage, as defined by elevated cTnI values, in asymptomatic ESRD patients on regular hemodialysis.
MATERIAL AND METHODS
This cross-sectional study was undertaken in the hemodialysis unit of Baskent University Hospital. The study was in accordance with the declaration of Helsinki, and written informed consent was obtained from all patients before enrollment. None of the patients had chest pain, electrocardiographic changes suggestive of arrhythmia, myocardial injury, or ischemia, except for left ventricular hypertrophy. None of the patients experienced intradialytic hypotension, cramps or a bleeding episode during the hemodialysis sessions within last four weeks. Patients with acute infections, active hepatitis, or non-specific fever, as well as those receiving nutritional support, were not included. Any patient suffering from acute coronary syndrome, myocardial infarction, angina pectoris, or coronary revascularization procedure (coronary stent replacement and coronary artery bypass graft surgery) and patients with a history of stroke, transient ischemic attack, carotid revascularization procedure, intermittent claudication, ischemic leg ulcer, peripheral vascularization, or amputation for critical limb ischemia within the last three months were excluded.
Baseline demographic, anthropometric, and clinical data including smoking history, body mass index, presence of diabetes mellitus, and use of antihypertensive medication and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) were recorded. Patients were considered as having atherosclerosis if at least one of the following clinical conditions were present: coronary artery disease (defined as previous myocardial infarction, angina pectoris, or coronary revascularization procedure), peripheral vascular disease (defined as previous intermittent claudication, ischemic leg ulcer, peripheral vascularization, or amputation for critical limb ischemia), cerebrovascular disease (defined as previous stroke, transient ischemic attack, or carotid revascularization procedure). All patients were evaluated by physical examination, and pre-dialysis blood pressure measurements were obtained. Dry weight was established for each patient on a trial-and-error basis and was defined as the weight below which the patient suffered frequent hypotensive episodes during the latter part of the dialysis session and experienced malaise, cramps, and dizziness postdialysis.
Uncontrolled hypertension was defined as pre-dialysis systolic blood pressure >140 mmHg and/or pre-dialysis diastolic blood pressure >90 mmHg. Electrocardiography was obtained before the hemodialysis session. Left ventricular hypertrophy was electrocardiographically determined by using Sokolow-Lyon voltage (sum of the amplitude of the S wave in lead V1 and the R wave in lead V5 or V6 >35 mm).Citation[12]
All patients were receiving four-hour hemodialysis thrice weekly with standard bicarbonate dialysis solution. The adequacy of dialysis was assessed by single pool Kt/V (spKt/V) using the following formula:where spKt/V is a single-pool Kt/V, R is the ratio of post dialysis to pre-dialysis blood urea nitrogen, t is time on dialysis in hours, UF is the amount of ultrafiltration in liters, and W is post-dialysis body weight in kilograms. The normalized protein equivalent of total nitrogen appearance (nPNA), also known as normalized protein catabolic rate, was calculated to estimate the daily protein intake by the following formula:
where C is the pre-dialysis concentration of blood urea nitrogen in milligrams per deciliter.
Body mass index was calculated as the ratio of dry weight in kilograms (end-dialysis weight) to height squared (in square meters).
Laboratory parameters including serum hemoglobin, glucose, albumin, prealbumin, C-reactive protein, blood urea nitrogen, creatinine, uric acid, calcium, phosphorus, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, iron, ferritin, transferrin, parathyroid hormone, cTnI, homocysteine, and tumor necrosis factor-alpha (TNF-α) were measured before the beginning of the hemodialysis session. Post-dialysis blood urea nitrogen concentration was used to calculate urea reduction ratio.
Cardiac troponin I was analyzed by a Microparticle Enzyme Immunoassay (Axsys System, Abbott Park, Illinois, USA) with a normal range of cTnI value from 0.02 to 2.3 ng/mL. The measuring range was 0.02–50 ng/mL. Cardiac troponin I was considered as elevated if the measurement was above the normal limit. Homocysteine was measured by fluorescent polarizing enzyme immunoassay (Axis Biomedicals ASA, Oslo, Norway) with a measuring range of 2–50 μmol/L, coefficient of variation: <10%, and normal laboratory range 5–15 μmol /L. Serum levels of TNF-α was measured with enzyme-linked immunosorbent assay method by using the Biosourche rat kit (Cat. no: KRC3011, Nivelles, Belgium). Other biochemical parameters were measured using standard laboratory methods.
Statistical Analysis
Statistical analysis was performed using SPSS 10.0 for Windows (SPSS Inc, Evanston, Illinois, USA). Results are considered statistically significant if two-tailed p is less than 0.05. Data are shown as mean ± standard deviation, median range and as a percentage (%). Comparisons between two groups were assessed by means of Mann Whitney-U test for continuous variables, as one of the groups was composed of 10 patients. Categorical variables were analyzed by the Fishers' exact test. Correlations were analyzed as either by Pearson (if two of the variables were normally distributed) or Spearman's correlation coefficients (if at least one of the variable was not normally distributed). A multiple linear regression analysis with backward elimination method was performed to identify possible independent predictors of cTnI. Variables included were age, sex, smoking status, diabetes mellitus, coronary artery disease, left ventricular hypertrophy, body mass index, uncontrolled blood pressure, nPNA, hemoglobin, albumin, HDL-C, LDL-C, triglyceride, homocysteine, C-reactive protein, and TNF-α. Because cTnI was not normally distributed, logarithmic conversion was performed before linear regression analysis.
RESULTS
In total, 113 hemodialysis patients (male/female, 66/47; mean age, 50.9 ± 11.9 years; mean hemodialysis duration, 99.7 ± 56.8 months) were included in the study. The etiologies for ESRD were glomerulonephritis in 21, hypertension 21, diabetes in 18, vesicourethral reflux/pyelonephritis in 16, nephrolithiasis in 10, polycystic kidney disease in 6, and amyloidosis in 4 patients. The etiology was unknown in 17 patients. Seventy-two (63.7%) patients were nonsmokers. Atherosclerosis was established in 46 patients (40.7%) and coronary artery disease in 38 (33.6%) patients. Peripheral artery disease and cerebrovascular diseases were present in 15 and 6 patients, respectively.
Cardiac troponin I concentrations were below the cutoff value (<2.3 ng/mL) in 103 (91.2%) patients (Group 1), whereas 10 (8.8%) patients had concentrations above the cutoff value (≥2.3 ng/mL) (Group 2). The comparative baseline characteristics, and demographic and clinical parameters of patients with normal and elevated cTnI are shown in and , respectively. When compared to Group 2, patients in Group 1 had higher hemoglobin and HDL-C levels and lower C-reactive protein and TNF-α levels (see ).
Table 1 Comparative baseline characteristics of hemodialysis patients with normal and elevated cardiac troponin I levels
Table 2 The comparative demographic and clinical parameters of hemodialysis patients with normal and elevated cardiac troponin I levels
Table 3 Comparative laboratory parameters of hemodialysis patients with normal and elevated cardiac troponin I levels
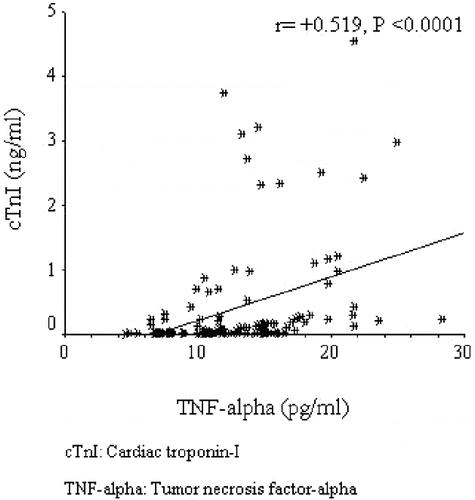
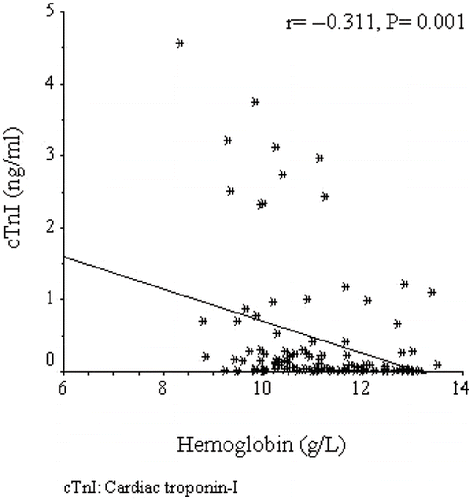
Cardiac troponin I was positively correlated with age (r = +0.223, p = 0.017), systolic blood pressure (r = +0.204, p = 0.03), and C-reactive protein (r = +0.425, p < 0.0001), and negatively correlated with albumin (r = −0.269, p = 0.004) and HDL-C (r = −0.295, p = 0.002) (data not shown). The regression graphics of cTnI with TNF-α, nPNA, and hemoglobin are shown in , respectively. An exploration of factors that may be associated with high log cTnI by backward multiple linear regression analysis revealed that diabetes mellitus, left ventricular hypertrophy, uncontrolled blood pressure, nPNA, hemoglobin, and TNF-α were found to be independently associated with logcTnI (see ).
Figure 2. The regression graphic between cardiac troponin I and normalized protein equivalent of total nitrogen appearance.
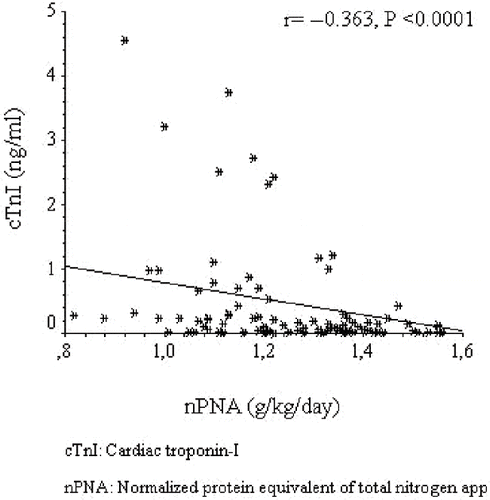
Table 4 Backward multiple linear regression analysis of factors that may be associated with high log cTnI
DISCUSSION
In ESRD patients, coronary artery disease is a major cause of death. Diagnosis of coronary artery disease in these patients is difficult, as many of these patients are asymptomatic and present with an abnormal electrocardiogram.Citation[3] As a marker of myocardial damage, cTnI is exclusively of cardiac originCitation[4] and is accepted as an indicator of myocardial micro-necrosis.Citation[13] The elevation of cTnI was shown to be independent of the magnitude of renal insufficiency, and no significant difference has been found between pre- and post-dialysis values. Therefore, cTnI is considered to be a sensitive and a specific marker of myocardial injury in ESRD patients, even in the absence of clinical, electrocardiographic, or echocardiographic pathologies.Citation[3,Citation4] In the present study, we searched for potential traditional and non-traditional risk factors in hemodialysis patients for cTnI elevation, which is considered to be a reliable marker of silent/subtle myocardial damage.
In our study, as a novel finding, we demonstrated significant correlation and independent relation between cTnI and TNF-α in clinically asymptomatic hemodialysis patients. Our findings are in accordance with the previous studies conducted in patients without renal failure.Citation[13,Citation14] There is a dual relationship between myocardial cell death (either due to necrosis or apoptosis) and the release of TNF-α. On one hand, myocardial necrosis triggers the release of TNF-α both from inflammatory cells and from myocytesCitation[15,Citation16]; on the other hand, TNF-α increases the rate of apoptotic cardiomyocyte death.Citation[17] In addition, during coronary arterial thrombosis and myocardial infarction, both TNF-α and troponin levels are increased.Citation[18] All of the above-mentioned evidence suggests that myocardial necrosis and the inflammatory marker, TNF-α, are interrelated. First of all, our findings confirm the interrelationship between cTnI and TNF-α levels in asymptomatic hemodialysis patients. Second, we have found that diabetes mellitus was independently associated with higher cTnI levels in hemodialysis patients. We do not know the mechanism behind the increased cTnI levels in diabetic ESRD patients. Previous studies have shown that diabetic patients had significantly higher levels cTnT levels compared to non-diabetics, and it was speculated that diabetic patients are at high risk for developing ischemic heart disease, which may be present but undiagnosed in this patient group.Citation[19,Citation20] In contrast to our findings, in one study, cTnI (unlike cTnT) was not associated with diabetes mellitus in hemodialysis patients. The authors speculated that the observed disparities in their findings may be attributed to the detection or release of complex forms, and to different half lives, catabolism pathways, and transient membrane permeability changes that occur during myocardial cell injury.Citation[11]. We believe that more studies are needed to highlight these conflicting issues.
In our study, despite its relatively low prevalence (38.9%), there was an independent and positive relationship between left ventricular hypertrophy (detected by electrocardiography) and logcTnI levels. Ilıou et al. also found that 35.8% of patients had left ventricular hypertrophy detected by echocardiography, and, unlike cTnI, cTnT was related with left ventricular hypertrophy.Citation[11] Tun et al. also did not find any relationship between cTnI and cardiac hypertrophy.Citation[21] However, the relationship between left ventricular hypertrophy and myocardial ischemia is well established and based on several mechanisms (e.g., coronary artery disease, or decrease in the perfusion of subendocardial wall).Citation[22] Martin et al. demonstrated that renal failure patients with elevated cTnI showed cardiac abnormalities demonstrated by echocardiography, nuclear imaging, or arteriography.Citation[23] Additionally, it was previously speculated that in cardiac hypertrophy, more ultrastructural components were synthesized with increased synthesis of enzymes, as well as an increased number and size of cardiac fibers. This may lead to increased leakage of cardiac specific enzymes.Citation[20] Missov et al. found increased serum cTnI levels in patients with congestive heart failure and speculated that the membranes of injured myocytes may lose their integrity and allow the exposure of intracellular myocytes.Citation[24]
Uncontrolled blood pressure was associated with high logcTnI levels in our study. Elevated blood pressures in dialysis patients may induce left ventricular hypertrophy, which in turn may be responsible for higher logcTnI levels in these patients. However, in our study, although patients with left ventricular hypertrophy had higher pre-dialysis systolic and diastolic blood pressures, these differences did not have a statistical significance (data not shown). Thus, we speculate that higher blood pressure per se had an impact on elevation of cTnI, independent of its effect on left ventricular hypertrophy. It is known that essential hypertension is a well-known risk factor for myocardial infarction.Citation[25] It was previously speculated that there was a link between cardiac ischemia and systemic hypertension, although the exact mechanisms were not known.Citation[26] One of the postulated mechanisms is the impairment of coronary endothelial dysfunction by hypertension. Presence of inflammationCitation[27] and oxidative stressCitation[28] may play a role for the endothelial dysfunction. Thus, hypertension apart from inducing ventricular hypertrophy can also induce endothelial dysfunction by various mechanisms and induce cardiac damage.
We found that one of the independent factors for higher cTnI was the presence of lower hemoglobin levels. Ritz et al. suggested that anemia may represent an important risk factor for accelerated atherosclerosis in dialyzed patients.Citation[29] It is well-known that higher hematocrit values were associated with lower cardiac and all cause mortality in incident hemodialysis patients, even in those with cardiac disease.Citation[30] The exact role of anemia in increasing mortality in dialysis patients is not completely understood.Citation[31] One explanation may be that the association between anemia and mortality risk may be mediated through cardiovascular disease, malnutrition, and inflammation. In our study, hemoglobin was positively correlated with albumin (r = +0.327, p < 0.0001) and prealbumin (r = +0.170, p = 0.026), and was negatively correlated with C-reactive protein (r = −0.251, p = 0.001) and TNF-α (r = −0.287, p < 0.0001) (data not shown). Erythropoietin resistance index, defined as the average weekly erythropoietin dose divided by the average blood hemoglobin value,Citation[32] was significantly higher in the high cTnI group. Albumin (r = −0.334, p < 0.0001) and prealbumin (r = −0.230, p = 0.02) were negatively correlated with erythropoietin resistance index, whereas C-reactive protein (r = +0.232, p = 0.02) and TNF-α (r = +0.442, p < 0.0001) were positively correlated with erythropoietin resistance index (data not shown). All of our findings support the well-known notion that protein-energy malnutrition, inflammation, and refractory anemia, which are considered as non-traditional risk factors leading to cardiovascular disease, are closely related in patients with ESRD.Citation[33] We can speculate that anemia, which is closely related with inflammatory and nutritional markers, mediate its adverse effects through cardiovascular disease reflected as high cTnI levels in our study population. However, in our study, anemia was associated with higher cTnI levels independent of other inflammatory and nutritional markers. We suggest that, apart from association with protein-energy malnutrition and inflammation, anemia is directly involved in myocardial damage in hemodialysis patients at least in two ways; by limiting oxygen delivery in the presence of atherosclerotic lesions and by its effect on cardiac structure through the induction of left ventricular hypertrophy.Citation[34]
In dialysis patients, nPNA reflects daily protein intake. Even in adequately dialyzed patients, nPNA is correlated with mortality.Citation[35] Not only baseline nPNA, but changes in nPNA over time are associated with survival in hemodialysis patients, independent of other nutritional and inflammatory markers. It is suggested that protein intake, as detected by nPNA, might be an independent factor for persistent cardiovascular disease and mortality in dialysis patients, with mechanisms being unclear.Citation[36] Although we did not analyze mortality, our present findings first showed that there was an independent association between nPNA and higher cTnI levels. As already mentioned earlier, in asymptomatic hemodialysis patients, higher cTnI levels at the time of presentation might be related with subclinical myocardial damage and later be associated with major cardiovascular disease. By the light of our findings, we can speculate that even in asymptomatic HD patients, low nPNA levels associated with cardiac damage could result in higher all cause and cardiovascular mortality, even with adjustments after inflammation and other nutritional factors.
We found no independent relationship between homocysteine and higher cTnI levels. Conflicting findings are present about the relationship between homocysteine and cardiovascular disease in hemodialysis patients. In one five-year follow-up period of the study, Kumagai et al. found no relationship between homocysteine and atherosclerotic indices and cardiovascular events.Citation[37] Also, it was suggested that a worsening of atherosclerotic cardiovascular disease is mostly related to malnutrition-inflammation cachexia syndrome, a condition in which homocysteine levels are low instead of high.Citation[38]
Caglar et al. showed that silent myocardial ischemia detected by single photon emission computed tomography in patients undergoing hemodialysis had higher homocysteine levels.Citation[39] Thus, the results for our patients does not fulfill the terms of traditional epidemiology and paradoxical reverse epidemiology of homocysteine.
We are aware that our study has limitations, and our findings should be interpreted with caution. Because our study is not an experimental one, we cannot prove a cause-and-effect relationship. Due to the cross-sectional design and the fact that blood samples were taken only once, we were unable to examine impact of anemia, nPNA, and inflammation on cTnI levels over time. We did not examine cardiac structural changes and cardiac function (e.g., cardiac hypertrophy, ejection fraction) by more reliable methods like echocardiography. We detected cardiac hypertrophy by electrocardiogram, which is less sensitive than echocardiography in this respect. We also did not analyze cardiac atherosclerosis by conventional methods like angiography, and the presence of cardiovascular disease was based on history and medical records. In our study, only 8.8% of patients had cTnI levels above cutoff. At first glance, this seems to be a very small number. However, our findings are consistent with previous literature. Katerinitis et al. found that cTnI levels were persistently elevated in 8% of asymptomatic hemodialysis patients, which is very similar to our findings.Citation[40] Finally, it should be emphasized that the results obtained in this study may not be applicable to all patient population because of the impact of differences, in age, sex, race, comorbidity, and so on.
In conclusion, to the best of our knowledge, we first demonstrated that both traditional and non-traditional risk factors are independently associated with higher cTnI levels. We suggest that traditional and non-traditional risk factors are not only associated with manifest disease, but they are also risk factors for silent myocardial damage in asymptomatic hemodialysis patients.
DECLARATION OF INTEREST
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Santoro A, Mancini E. Cardiac effects of chronic inflammation in dialysis patients. Nephrol Dial Transplant. 2002;17:10–15.
- Yao Q, Pecoits-Filho R, Lindholm B, Stenvinkel P. Traditional and non-traditional risk factors as contributors to atherosclerotic cardiovascular disease in end-stage renal disease. Scand J Urol Nephrol. 2004;38:405–416.
- Apple FS, Sharkey SW, Hoeft P, Skeate R, Voss E, Dahlmeier BA, Prognostic value of serum cardiac troponin I and T in chronic dialysis patients: A one-year outcomes analysis. Am J Kidney Dis. 1997;29:399–403.
- Khan IA, Wattanasuwan N, Mehta NJ, Tun A, Singh N, Singh HK, Prognostic value of serum cardiac troponin I in ambulatory patients with chronic renal failure undergoing long-term hemodialysis: A two-year outcome analysis. J Am Coll Cardiol. 2001;38:991–998.
- Wayand D, Baum H, Schätzle G, Schärf J, Neumeier D. Cardiac troponin T and I in end-stage renal failure. Clin Chem. 2000;46:1345–1350.
- Schulz O, Sigusch HH. Impact of an exercise-induced increase in cardiac troponin I in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;90:547–550.
- Roberts MA, Fernando D, Macmillan N, Proimos G, Bach LA, Power DA, Single and serial measurements of cardiac troponin I in asymptomatic patients on chronic hemodialysis. Clin Nephrol. 2004;61:40–46.
- Beciani M, Tedesco A, Violante A, Cipriani S, Azzarito M, Sturniolo A, Cardiac troponin I (2nd generation assay) in chronic hemodialysis patients: Prevalence and prognostic value. Nephrol Dial Transplant. 2003;18:942–946.
- Stoffel MP, Pollok M, Baldamus CA. Troponin I is a better prognostic parameter of cardiovascular events in asymptomatic patients on hemodialysis than troponin T. Nephrol Dial Transplant. 2000;15:1259–1260.
- Troyanov S, Ly QH, Schampaert E, Ammann H, Lalumière G, Madore F, Diagnostic specificity and prognostic value of cardiac troponins in asymptomatic chronic haemodialysis patients: A three year prospective study. Heart. 2005;91:1227–1228.
- Iliou MC, Fumeron C, Benoit MO, Tuppin P, Courvoisier CL, Calonge VM, Chronic Haemodialysis and New Cardiac Markers Evaluation (CHANCE) Study. Factors associated with increased serum levels of cardiac troponins T and I in chronic haemodialysis patients. Chronic Hemodialysis and New Cardiac Markers Evaluation (CHANCE) study. Nephrol Dial Transplant. 2001;16:1452–1458.
- Konno T, Shimizu M, Ino H, Fujino N, Hayashi K, Uchiyama K, Differences in diagnostic value of four electrocardiographic voltage criteria for hypertrophic cardiomyopathy in a genotyped population. Am J Cardiol. 2005;96:1308–1312.
- Knebel F, Schimke I, Eddicks S, Walde T, Ziebig R, Schattke S, Does contrast echocardiography induce increases in markers of myocardial necrosis, inflammation and oxidative stress suggesting myocardial injury?. Cardiovasc Ultrasound. 2005;17:3–21.
- Cusack MR, Marber MS, Lambiase PD, Bucknall CA, Redwood SR. Systemic inflammation in unstable angina is the result of myocardial necrosis. J Am Coll Cardiol. 2002;39:1917–1923.
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47.
- Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Tumor necrosis factor receptor signaling resistance in the female myocardium during ischemia. Circulation. 2006;114:I282–1289.
- Kubota T, Miyagishima M, Frye CS, Alber SM, Bounoutas GS, Kadokami T, Overexpression of tumor necrosis factor-alpha activates both anti- and pro-apoptotic pathways in the myocardium. J Mol Cell Cardiol. 2001;33:1331–1344.
- Antonicelli R, Olivieri F, Cavallone L, Spazzafumo L, Bonafè M, Marchegiani F, Tumor necrosis factor-alpha gene-308G. A polymorphism is associated with ST-elevation myocardial infarction and with high plasma levels of biochemical ischemia markers. Coron Artery Dis. 2005;16:489–493.
- Löwbeer C, Stenvinkel P, Pecoits-Filho R, Heimbürger O, Lindholm B, Gustafsson SA, Elevated cardiac troponin T in predialysis patients is associated with inflammation and predicts mortality. J. Intern Med. 2003;253:153–160.
- Löwbeer C, Ottosson-Seeberger A, Gustafsson SA, Norrman R, Hulting J, Gutierrez A. Increased cardiac troponin T and endothelin-1 concentrations in dialysis patients may indicate heart disease. Nephrol Dial Transplant. 1999;14:1948–1955.
- Tun A, Khan IA, Win MT, Hussain A, la TA H, Wattanasuwan N, Specificity of cardiac troponin I and creatine kinase-MB isoenzyme in asymptomatic long-term hemodialysis patients and effect of hemodialysis on these cardiac markers. Cardiology. 1998;90:280–285.
- Solaro RJ. Troponin I, stunning, hypertrophy, and failure of the heart. Circ Res. 1999;84:122–124.
- Martin GS, Becker BN, Schulman G. Cardiac troponin-I accurately predicts myocardial injury in renal failure. Nephrol Dial Transplant. 1998;13:1709–1712.
- Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation. 1997;96:2953–2958.
- Mashimo Y, Suzuki Y, Hatori K, Tabara Y, Miki T, Tokunaga K, Association of TNFRSF4 gene polymorphisms with essential hypertension. J Hypertens. 2008;26:902–913.
- Bacaner M, Brietenbucher J, LaBree J. Prevention of ventricular fibrillation, acute myocardial infarction (myocardial necrosis), heart failure, and mortality by bretylium: Is ischemic heart disease primarily adrenergic cardiovascular disease?. Am J Ther. 2004;11:366–411.
- Naya M, Tsukamoto T, Morita K, Katoh C, Furumoto T, Fujii S, Plasma interleukin-6 and tumor necrosis factor-alpha can predict coronary endothelial dysfunction in hypertensive patients. Hypertens Res. 2007;30:541–548.
- Lip GY, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG. Oxidative stress in malignant and non-malignant phase hypertension. J Hum Hypertens. 2002;16:333–336.
- Ritz E. Atherosclerosis in dialyzed patients. Blood Purif. 2004;22:28–37.
- Li S, Collins AJ. Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodialysis patients. Kidney Int. 2004;65:626–633.
- Duke M, Abelmann WH. The hemodynamic response to chronic anemia. Circulation. 1969;39:503–515.
- Gunnell J, Yeun JY, Depner TA, Kaysen GA. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 1999;33:63–72.
- Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263.
- Levin A. The role of anaemia in the genesis of cardiac abnormalities in patients with chronic kidney disease. Nephrol Dial Transplant. 2002;17:207–210.
- Kalantar-Zadeh K, Supasyndh O, Lehn RS, McAllister CJ, Kopple JD. Normalized protein nitrogen appearance is correlated with hospitalization and mortality in hemodialysis patients with Kt/V greater than 1.20. J Ren Nutr. 2003;13:15–25.
- Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis. 2006;48:37–49.
- Kumagai H, Sakurai M, Takita T, Maruyama Y, Uno S, Ikegaya N, Association of homocysteine and asymmetric dimethylarginine with atherosclerosis and cardiovascular events in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:797–805.
- Kalantar-Zadeh K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next?. Semin Dial. 2005;18:365–369.
- Caglar M, Mahmoudian B, Aytemir K, Kahraman S, Arici M, Kabakci G, Value of 99mTc-methoxyisobutylisonitrile (99mTc-MIBI) gated SPECT for the detection of silent myocardial ischemia in hemodialysis patients: Clinical variables associated with abnormal test results. Nucl Med Commun. 2006;27:61–69.
- Katerinis I, Nguyen Q, Magnin JL, Descombes E. Cardiac findings in asymptomatic chronic hemodialysis patients with persistently elevated cardiac troponin I levels. Ren Fail. 2008;30:357–362.