Abstract
Augmenter of liver regeneration (ALR) enhances the proliferation of hepatocytes and accelerates recovery from acute liver failure in animal models. ALR is expressed in both the liver and kidney; however, the specific location of ALR expression and its biological effects in the kidney remain unknown. We aimed to investigate the efficacy of ALR in rats with gentamicin (GM)-induced acute renal failure (ARF). Rats were randomized into the normal group, GM+saline group, GM+vehicle group, and GM+rrALR group. Blood urea nitrogen, serum creatinine, and urine beta-N-acetyl-D-glucosaminidase were measured, and histological analyses of the kidneys were performed. The expression of ALR protein was determined by immunohistochemistry and Western blotting. In vitro incorporation of tritiated thymidine was used to measure the proliferation of renal tubular epithelial cells. In normal rats, the expression of ALR protein was faint in the medulla and absent in the cortex. However, in ARF rats, ALR expression increased significantly in both the renal cortex and medulla. Histological analyses revealed that treatment with recombinant rat ALR (rrALR) reduced the extent of injury of tubular cells in the renal cortex. Serum/urine biochemical parameters also showed that renal dysfunction was improved by the administration of rrALR. Intraperitoneal injection of rrALR enhanced the proliferation of tubular cells in vivo. We also confirmed that rrALR could promote the proliferation of renal tubular cells in vitro. These results indicate that rrALR effectively accelerates kidney recovery after ARF induced by gentamicin, and that the protective effect is associated with enhanced proliferation of renal tubular cells.
INTRODUCTION
Acute renal failure (ARF) is a serious disorder with a high morbidity and mortality. Although the pathophysiological mechanisms of ARF have been studied extensively, the blood purification and supporting therapies that are used to treat the disorder do not stimulate the restoration of renal tubular cells effectively. Therefore, the mortality rate still remains high, up to 50%.Citation[1,Citation2] Recently, a number of studies have confirmed that several growth factors can promote the restoration of renal function by accelerating the proliferation of renal tubular epithelial cells; this indicates that it might be possible to use growth factors to cure ARF.Citation[3–5]
Hagiya et al.Citation[6] purified and cloned the growth factor augmenter of liver regeneration (ALR), a 15 kD protein, from the liver tissue of newborn rats in 1994. ALR is a heat-stable and non-specific factor that promotes the regeneration of liver cells. It differs from hepatocyte growth factor (HGF) in both its gene sequence and protein structure. Recent studies have found that ALR can significantly stimulate DNA synthesis in hepatocytes after partial hepatectomy and can improve the survival rate of rats with liver failure caused by carbon tetrachloride (CCl4).Citation[7,Citation8] ALR is expressed in both the liver and kidney. However, the specific location of ALR expression in the kidney and its biological effects are not clear, and so far the related studies have not been seen in the literature.
To evaluate the biological effects of ALR in the kidney, we examined whether ALR was involved in the pathological process of ARF by determining its expression pattern in the kidneys of normal and ARF rats. In addition, the effects of recombinant rat ALR (rrALR) on renal functional impairment and histological damage were determined by injection of the exogenous protein into ARF rats.
MATERIALS AND METHODS
Main Reagents
The gentamicin (GM) that was used in vivo and in vitro was provided by Southwest Pharmaceutical Co., Ltd. (Chongqing, China) and Sigma Co., Ltd. (St. Louis, Missouri, USA), respectively. Supernatant of expression products of empty plasmid, rrALR, and rabbit anti-rat ALR polyclonal antibody have been described by us previously.Citation[9] The mouse monoclonal antibody against rat proliferating cell nuclear antigen (PCNA) was purchased from R&D Systems (Minneapolis, Minnesota, USA). Horse radish peroxidase (HRP)-tagged goat anti-rabbit IgG was from Santa Cruz Co. (Santa Cruz, California, USA). Tritiated thymidine ([3H]TdR) was provided by the Chinese Atomic Energy Research Establishment (AERE; Beijing, China).
Establishment of the Animal Model and Groups Utilized
One hundred and twenty male Sprague-Dawley (SD) rats that weighed 200–250 g were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China) and kept in individual cages in an experimental room at a temperature of 22–28°C. They were given free access to standard food and water. All experimental procedures conformed to institutional Guidelines for the Care and Use of Laboratory Animals in Chongqing Medical University, Chongqing, China, and also conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996). The animals were divided randomly into four groups (30 rats/group) at 1 w after the adaptive feeding:
normal group: injected with saline once daily for 7 days and then received a further daily injection of saline for 14 days;
GM+saline group: injected with gentamicin (140 mg/kg/d) for 7 days and then injected with saline daily for 14 days;
GM+vehicle group: injected with GM (140 mg/kg/d) for 7 days and then with vehicle (products of expression from the empty plasmid) for 14 days; and
GM+rrALR group: injected with GM (140 mg/kg/d) for 7 days and then with rrALR (80 μg/kg/d) for 14 days.
The daily saline, vehicle, and rrALR intraperitoneal injections were of equal volume and commenced on the same day. Samples of urine and blood, together with kidneys, were collected on days 4, 8, 12, 16, and 21.
Determination of Renal Function
Total urine output was collected for 24 hours before the animals were sacrificed. N-acetyl-beta-D-glucosaminidase (NAG) in the urine was detected according to the classical approach.Citation[10] The rat eyeballs were picked off to acquire the blood samples. The blood samples were centrifuged and serum was collected. A 747-autoanalyzer (Hitachi Co., Ltd, Tokyo, Japan) was employed to determine the levels of blood urea nitrogen (BUN) and serum creatinine (Scr).
Histological Examination
Fresh kidneys were washed gently with phosphate-buffered saline (PBS; 0.01 M). They were cut longitudinally and fixed with neutral formalin (10%). Tissue was embedded in paraffin wax, and 3-μm sections were stained by the conventional periodic acid-Schiff (PAS) technique.
Immunohistochemistry
The paraffin slices (D = 3 μm) were deparaffinized and hydrated conventionally. Methanol with 3% H2O2 was used to block the endogenous peroxidase followed by heating in a microwave for retrieving antigens. The sections were first blocked with normal goat serum (Sigma, St. Louis, Missouri, USA), then either the rabbit anti-rat ALR polyclonal antibody or mouse anti-rat PCNA monoclonal antibody were added respectively, incubating overnight at 4°C. After this, the corresponding secondary antibody conjugated to horseradish peroxidase (HRP) was added. The dehydration was performed after DAB staining and hematoxylin counterstaining. The sections were mounted after cleaning with dimethylbenzene. Negative controls were performed using normal rabbit serum or PBS instead of the primary antibody for ALR and PCNA immunohistochemistry, respectively. Twenty successive non-duplicated fields (×400) were selected randomly, and the number of renal tubular cells in which positive expression was observed was counted.
Quantification of ALR Protein Expression by Western Blot
Kidneys (100 mg) were shred and washed twice with PBS. An aliquot of 1 ml of cell lysis solution (20 mmol/l Tris-HCl buffer pH 7.5, 0.15 mol/l NaCl, 1 mol/l EDTA, 1% Triton X-100, and 150 μg/mL PMSF) was added to extract the total protein. The concentration of total protein was determined by staining with Coomassie Brilliant Blue. Protein samples (100 μg) were subjected to electrophoresis through a 15% polyacrylamide gel and then transferred onto a polyvinylidene difluoride (PVDF) membrane at constant voltage. Then, the PVDF membrane was blocked with 5% dried skim milk in PBS with Tween 20 (PBST) and incubated for 1 h at room temperature after the addition of the anti-ALR polyclonal antibody (1:500). After washing with PBST, the membrane was incubated for 1 h with HRP-tagged goat anti-rabbit IgG. Proteins were visualized using chemiluminescence. β-actin was used as an internal control in each membrane.
Cell Culture and Treatments
The rat renal tubular epithelial cell line HK2 (ATCC, Manassas, Virginia, USA) was grown in Dulbecco's modified Eagle's medium (DMEM)/F12 medium supplemented with 10% fetal calf serum (FCS) (HyClone, Logan, Utah, USA). For all experiments, the cells were maintained in flasks at 37°C in a humidified atmosphere that contained 5% CO2/95% air. For treatment with different factors, the HK2 cells were divided randomly into the following four groups: the vehicle group (incubated with the expression products of the empty plasmid), rrALR group (2.5 ng/mL–50 μg/mL rrALR), GM group (5 mg/mL GM), and GM+rrALR group (5 mg/mL GM and different doses of rrALR (i.e., 10, 25, and 50 μg/mL)).
Measurement of HK2 Cell Proliferation by the Incorporation of [3H]TdR
HK2 cells were seeded in 96-well plates at 4 × 103 cells/well and incubated for 12 h in DMEM/F12 supplemented with 10% FCS. The cells were then incubated in fresh serum-free DMEM/F12 for 24 h. The cells were treated with different media as mentioned above. After incubation for 12–96 h, [3H]TdR (1 μCi/well) was added to each well. The culture medium was removed after incubation for an additional 12 h. Cells were washed gently once with PBS and trypsinogen was added to digest the cells. The amount of incorporation of [3H]TdR was determined using an isotope scanner after the cells had been harvested.
Statistical Analysis
All the data were expressed as the mean ± SEM. The statistical analysis software SPSS 10.0 was utilized in this study. The Student's t-test was used to examine the differences between two groups, and one-factor analysis of variance (ANOVA) was employed to examine the differences among several groups. A p value of less than 0.05 was considered statistically significant.
RESULTS
Detection of ALR Protein Expression in Renal Tissues
Expression of Endogenous ALR Protein in Normal and ARF Rats
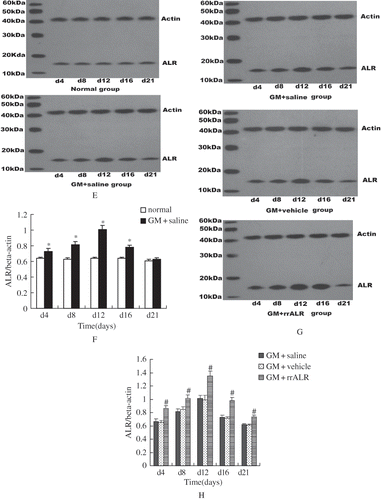
In normal rats, no ALR expression was detected in the renal cortex (see ). However, in ARF rats, ALR expression in the cortex was increased remarkably, especially in the impaired and regenerative proximal tubular cells (see ). In the renal medulla, there was faint expression of ALR in the loop of Henle, distal tubule, and collecting duct in normal rats (see ). Expression of ALR in the medulla was also increased significantly in ARF rats (see ). No ALR expression was detected in the glomerulus of normal or ARF rats. Western blotting showed that ALR protein in ARF rats (GM+saline group) began to increase significantly from day 4, reached a peak on day 12, started to decrease on day 16 (p < 0.05), and had almost returned to the normal level on day 21 (p > 0.05; see and ).
Figure 1A–D. ALR expression in rat kidneys. No ALR expression could be detected in the renal cortex of normal rats (A). However, ALR expression in the cortex of GM+saline rats was increased markedly (day 8) (B). In the renal medulla, faint expression of ALR was detected in normal rats (C). Expression in the medulla was increased significantly in GM+saline rats (SABC, ×200) (D).
Changes in the Level of ALR Protein in Renal Tissue after Exogenous Injection of rrALR
Similarly to the GM+saline group, renal ALR protein in the GM+rrALR group increased significantly on day 4, reached a peak on day 12, and then decreased from day 16. However, the levels of ALR protein in the kidneys of rats in the GM+rrALR group were much higher than those in the GM+vehicle group and GM+saline group at the above-mentioned time points (p < 0.05; see and ). No significant differences in the levels of ALR protein were found between the GM+saline and GM+vehicle groups (p > 0.05).
Effects of rrALR on Renal Function in ARF Rats
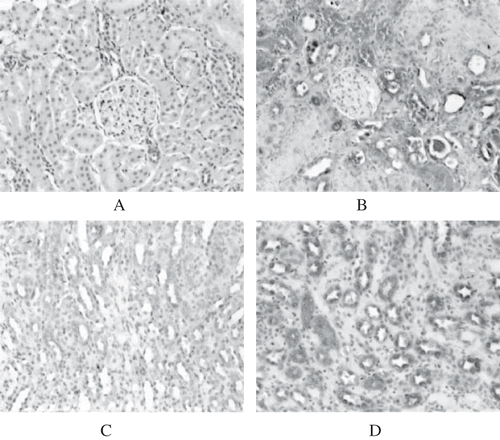
The levels of BUN (see ), Scr (see ), and urinary NAG (see ) in the GM+rrALR, GM+saline, and GM+vehicle groups were markedly higher on days 4, 8, 12, and 16 than those in the normal group (p < 0.05). No significant differences were observed between the GM+saline and GM+vehicle groups at the above-mentioned time points (p > 0.05). However, the increases in BUN, Scr, and urinary NAG on days 4, 8, 12, and 16 after GM administration were less marked in the GM+rrALR group as compared with the vehicle group (p < 0.05). These biochemical data had returned to normal levels on day 21 in all groups (p > 0.05).
Figure 2. Effects of rrALR on renal function in ARF rats. Levels of BUN (A), Scr (B), and urinary NAG (C) in the GM+rrALR group were significantly lower that those in the GM+vehicle group at the same timepoints. There was no significant difference in renal function between GM+saline rats and GM+vehicle rats. All data are shown as the mean ± standard deviation. *p < 0.05 compared with the normal group at the same time point; #p < 0.05 vs. the GM+vehicle group at the same time point.
Effects of rrALR on the Pathological Changes in ARF Rats
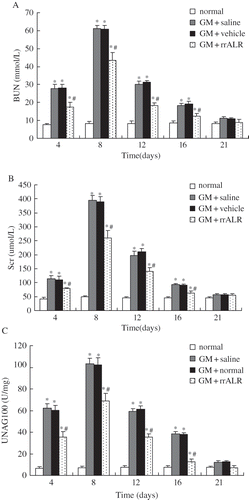
In normal rats, periodic acid Schiff (PAS) staining revealed an integrated renal tubular basement membrane and a distinct structure of the renal tubular epithelial cells, with no degeneration or necrosis, and no intratubular casts (see ). However, severe degeneration, disintegration, desquamation, and luminal congestion of the renal tubular epithelial cells with loss of the brush border could be seen in GM+saline rats (see ) and GM+vehicle rats (see ) on days 4, 8, and 12. The great bulk of protein casts, renal interstitial dropsy, and accompanying infiltration of inflammatory cells could also be seen. These pathological changes were mitigated on day 16, and they had almost returned to normal on day 21. In contrast, renal sections obtained from rats treated with rrALR (GM+rrALR group) demonstrated a marked reduction in the histological features of renal injury; this corresponded to fewer casts, lower levels of mesenchymal dropsy, and less inflammatory cell infiltration (see ).
Figure 3. PAS-stained sections of rat kidneys on day 8. The sections from normal rats displayed an integrated renal tubular basement membrane and distinct structure of the renal tubular epithelial cells (A). The characteristic features of nephrotoxic ARF were evident in the cortexes of GM+saline rats (B) and GM+vehicle rats (C). There were no significant differences in histological changes between the GM+saline and GM+vehicle rats. However, treatment with rrALR decreased the amount of tubular lesions significantly (D) (PAS, ×200).
Effects of rrALR on the Proliferation of Renal Tubular Cells in ARF Rats
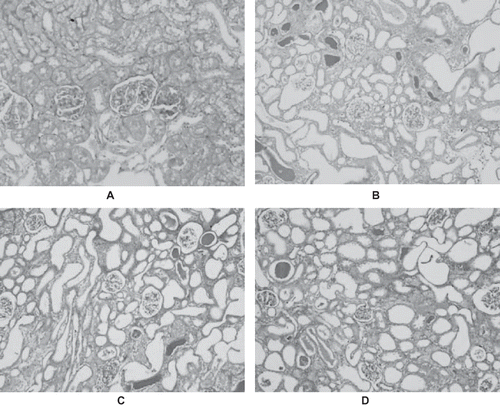
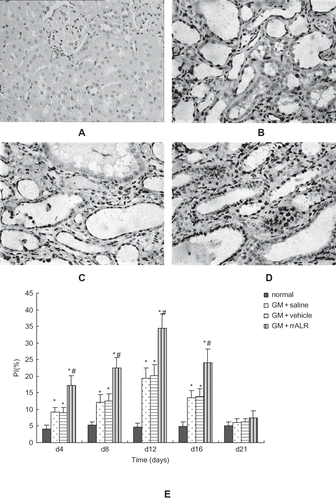
Few PCNA-positive particles could be seen in the nuclei of renal tubular epithelial cells in normal rats under the light microscope (see ). In GM+saline and GM+vehicle rats, PCNA-positive cells were distributed diffusely on day 8 (see and ), and this distribution was even more diffuse in GM+rrALR rats (see ) as compared to vehicle-treated rats. The proliferation index (PI) values, which reflected the proliferation of renal tubular epithelial cells, were lower in the normal group than in any other group (p < 0.05). The PI values for the GM+rrALR group were significantly higher than those for the GM+saline and GM+vehicle groups at each time point (p < 0.05). No significant difference in PI values was found between the GM+saline and GM+vehicle groups (p > 0.05) (see ).
Figure 4. Effects of rrALR on renal tubular cellular proliferation in rats. PCNA-positive tubular cells were seldom observed in normal rats (A). The number of PCNA-positive tubular cells increased after the administration of GM in the GM+saline (B) and GM+vehicle (C) groups. Intraperitoneal injection of rrALR enhanced the number of PCNA-positive tubular cells significantly (D) as compared with the GM+vehicle group (SABC, ×400). There was no significant difference in PI between the GM+saline and GM+vehicle rats (E). Data are shown as the mean ± SEM. *p < 0.05 vs. the normal group; #p < 0.05 vs. the GM+vehicle group.
Proliferative Effect of rrALR on HK2 Cells
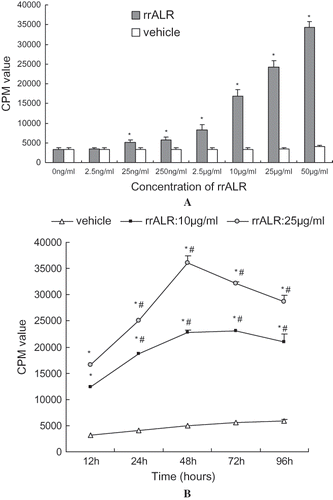
The effect of rrALR on the proliferation of HK2 cells was assessed by measuring the incorporation of [3H]TdR as an index of DNA synthesis. As shown in , the incubation of HK2 cells with various concentrations of rrALR for 24 h resulted in a dose-dependent increase in thymidine incorporation, which was maximal at 50 μg/mL (p < 0.01). However, 2.5 ng/mL rrALR exhibited no significant effect on cell proliferation (p > 0.05). Incubation of HK2 cells with 25 or 50 μg/mL rrALR over a period of 12 h to 96 h showed that rrALR could increase thymidine incorporation as early as 12 h, and peak incorporation was reached at 48 h (see ).
Figure 5. Proliferative effect of rrALR on HK2 cells in vitro. Confluent and quiescent HK2 cells were incubated with the vehicle or different concentrations of rrALR (2.5 ng/ml–50 μg/ml) for 24 h. rrALR stimulated the proliferation of HK2 cells in a concentration-dependent manner (A). Confluent and quiescent HK2 cells were incubated with the vehicle or rrALR (10 μg/ml or 25 μg/ml) for 12 to 96 h. rrALR stimulated the proliferation of HK2 cells in a time-dependent manner (B). DNA synthesis was assessed by thymidine incorporation, and the results were expressed as CPM. Data are shown as the mean ± SEM. * p < 0.05 vs. the control group; #p < 0.05 vs. the same group at 12 h.
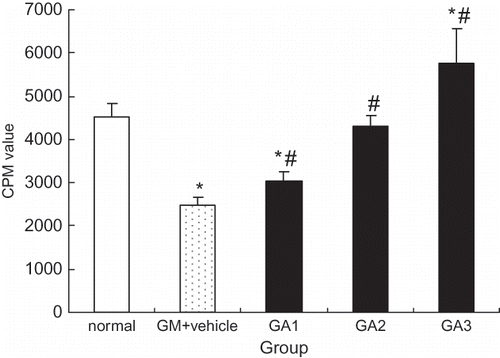
Incubation of HK2 cells with 5.0 mg/mL gentamicin for 24 h decreased the amount of thymidine incorporation dramatically, as described previously. In contrast, as shown in , thymidine incorporation increased significantly (p < 0.05), in a concentration-dependent manner, when HK2 cells were incubated with both gentamicin (5.0 mg/mL) and rrALR (10, 25, or 50 μg/mL).
Figure 6. rrALR stimulates mitogenesis in gentamicin-injured HK2 cells. Incubation of HK2 cells with 5.0 mg/ml gentamicin for 24 h resulted in a dramatic reduction in thymidine incorporation (GM+vehicle). In contrast, thymidine incorporation increased significantly in a dose-dependent manner when HK2 cells were incubated with both 5.0 mg/ml gentamicin and 10, 25, or 50 μg/ml rrALR (GA1, GA2, and GA3, respectively). DNA synthesis was assessed by thymidine incorporation, and the results are expressed as CPM. Data represent the mean ± SEM. *p < 0.05 vs. the normal group; #p < 0.05 vs. the GM+vehicle group.
DISCUSSION
It is generally accepted that ARF is caused by acute toxic injury, and that necrosis of renal tubular epithelial cells can be induced by aminoglycosides and some other drugs. Timely diagnosis and treatment play a key role in the successful treatment of the illness. However, regenerative tubular epithelial cells are required to replace the impaired and necrotic cells in order to restore renal function in the patient and maintain the integrity of epithelial structure and function. Thus, the ability to reduce the damage to tubular epithelial cells and promote their regeneration has become a key goal of recent studies.Citation[11–13]
In 1994, HagiyaCitation[6] found that ALR is expressed in the liver and in other tissues, such as the testes, kidney, and cerebrum, by northern blotting. Researchers have found that ALR is expressed at lower levels in normal liver tissue than in the liver of patients with cirrhosis of the liver.Citation[14] Furthermore, the degree of liver cirrhosis improved significantly after the injection of a plasmid that encoded ALR into rats with cirrhosis of the liver.Citation[15] The expression of ALR increased when acute hepatic failure occurred. Injection of a plasmid that encoded ALR into rats with acute liver failure resulted in decreased mortality due to hepatic failure, promotion of liver regeneration, and the decreased release of aspartate aminotransferase (AST) and alanine aminotransferase (ALT).Citation[7] The expression of ALR is increased in liver carcinoma.Citation[14] Moreover, it was confirmed that ALR could accelerate the proliferation of hepatic carcinoma cells.Citation[16] However, the specific location of ALR expression in the kidney and its biological effects has not been elucidated.
For this purpose, we observed the expression of ALR in the renal tissue of normal and ARF rats. The results indicated that ALR was expressed in the loop of Henle, distal convoluted tubule, and collecting duct in the renal medulla but not in the cortex. This also confirmed that the medulla is the area in which many growth factors are produced and stored. For example, epidermal growth factor (EGF) is expressed in the thick ascending limb of the loop of Henle and in the distal convoluted tubule,Citation[17,Citation18] whereas insulin-like growth factor 1 (IGF-1) is expressed mainly in the collecting duct.Citation[19]
However, it is the proximal tubule that is most susceptible to ischemia, hypoxia, and substances that are toxic to kidneys. Therefore, the cells of the proximal tubule have many receptors for growth factors that promote regeneration, such as EGF, IGF-1, and HGF.Citation[18–20] These growth factors affect renal tubular epithelial cells by both paracrine and autocrine binding to their receptors. During the process of ARF, the expression of ALR increased significantly in the tubular cells of the cortex, and ALR expression in the medulla also changed. The levels of ALR reached a peak on day 12 after the induction of ARF, and renal function began to recover after day 12 with a reduction in pathological lesions and significant tubule regeneration. This suggested that ALR might be related to renal regeneration and repair. ALR, in addition to other growth factors, could be involved in the pathological changes that occur during ARF through both paracrine and autocrine signaling.
To investigate the effects of ALR on ARF, we injected rrALR intraperitoneally into rats with GM-induced ARF. Western blot analysis was used to compare the levels of ALR protein in the kidney. The results showed that the level of ALR protein in the kidney increased significantly in rats that had been injected with exogenous rrALR, which suggested that intraperitoneal injection of rrALR could enhance the level of renal ALR. In this study, we confirmed that levels of BUN, Scr, and urinary NAG increased in rats that had been injected intraperitoneally with rrALR compared with those injected with saline or products of the empty plasmid. This indicated that rrALR could improve the renal function of ARF rats significantly.
Compared with the sections from the rats injected with vehicle, the sections from the rats injected with rrALR showed extenuated pathological damage. This included a reduction in protein casts, lower levels of interstitial edema, and comparatively less infiltration of inflammatory cells at the same time points. Obvious tubular cell regeneration with larger epithelial nuclei, more abundant and thicker chromatin, less cytoplasm, flat cells, expanded lumina, and a disorganized arrangement of cells was apparent in GM+rrALR rats on day 12 as compared to vehicle-treated rats. This suggested that rrALR promoted the regeneration and restoration of renal tubular epithelial cells.
To understand the relationship between rrALR and renal regeneration and restoration, we analyzed the expression of PCNA in renal tissue from the different groups. The expression of PCNA is related directly to DNA duplication and cell proliferation because it is a nuclear protein. In this study, we found that the number of cells that stained positive for PCNA was increased significantly in the GM+rrALR group as compared with the GM+vehicle group. The level and range of renal ALR expression increased simultaneously, which indicated that the proliferation of renal tubular cells was related closely to the local level of ALR. The expression of ALR increased during ARF. Consequently, we speculated that the increased production of ALR protein in the kidney after acute renal injury might affect the impaired tubular cells by means of autocrine or paracrine signaling, and this could have a therapeutic effect.
Does ALR have a direct effect on the proliferation of renal tubular epithelial cells? We found that rrALR did have a direct proliferative effect, which was concentration- and time-dependent, on HK2 cells that were cultured conventionally in vitro. This revealed that ALR might affect the regeneration of renal tubular epithelial cells, and help maintain the normal structure and function of renal tubules through the paracrine pathway in the normal physiological state. We also observed the effects of rrALR on HK2 cell proliferation in a pathological state. We found that GM could suppress the proliferation of HK2 cells, which was consistent with the results obtained in vivo. However, rrALR promoted the proliferation of HK2 cells after they had been impaired by treatment with GM. Furthermore, this effect became more significant as the concentration of rrALR was increased. These results demonstrated that rrALR also enhanced the regeneration of renal tubular epithelial cells in an acute impaired state, and explained why the proliferation of renal tubular epithelial cells increased significantly after the injection of exogenous rrALR.
In this study, we found, for the first time, that ALR was involved in the pathological process of ARF induced by GM. The pathological lesions could be attenuated and restoration of renal function could be promoted by increasing the concentration of ALR protein in the renal tissue of rats with ARF by the injection of exogenous rrALR. In additional experiments, we confirmed that ALR could accelerate the proliferation of renal tubular cells, which suggested that ALR plays a protective role in GM-induced ARF by promoting the proliferation of renal tubular epithelial cells.
ACKNOWLEDGMENTS
This work was supported by a grant from the Ph.D. Programs Foundation of the Ministry of Education of China (No. 20050631006) and grants from the Applied Basic Research Programs of the Public Health Bureau Foundation of Chongqing Province (No. 2008-1-44, 2008-2-221).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Waikar SS, Curhan GC, Ayanian JZ, Chertow GM. Race and mortality after acute renal failure. J Am Soc Nephrol. 2007;18:2740–2748.
- Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40:275–279.
- Cantaluppi V, Biancone L, Romanazzi GM, Macrophage stimulating protein may promote tubular regeneration after acute injury. J Am Soc Nephrol. 2008;19:1904–1918.
- Vesey DA, Cheung C, Pat B, Endre Z, Gobé G, Johnson DW. Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant. 2004;19:348–355.
- Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–4361.
- Hagiya M, Francavilla A, Polimeno L, Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: Expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA. 1994;91:8142–8146.
- Zhang LM, Liu DW, Liu JB, Effect of naked eukaryotic expression plasmid encoding rat augmenter of liver regeneration on acute hepatic injury and hepatic failure in rats. World J Gastroenterol. 2005;11:3680–3685.
- Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol. 2000;35:112–119.
- Liu Q, Yu H-F, Sun H, Ma HF. Expression of human augmenter of liver regeneration in pichia pastoris yeast and its bioactivity in vitro. World J Gastroenterol. 2004;10:3188–3190.
- Bourbouze R, Dubois M, Gluckman JC, Legrain M. Excretion of urinary N-acetyl-beta-D-glucosaminidase isoenzymes after renal transplantation in the rat. J Clin Chem Clin Biochem. 1987;25:71–76.
- Cheng CW, Ka SM, Yang SM, Nephronectin expression in nephrotoxic acute tubular necrosis. Nephrol Dial Transplant. 2008;23:101–109.
- Price PM, Megyesi J, Safirstein RL. Cell cycle regulation: Repair and regeneration in acute renal failure. Kidney Int. 2004;66:509–514.
- Nigam S, Lieberthal W. Acute renal failure, III: The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol. 2000;279:F3–F11.
- Thasler WE, Schlott T, Thelen P, Expression of augmenter of liver regeneration (ALR) in human liver cirrhosis and carcinoma. Histopathology. 2005;47:57–66.
- Li Q, Liu DW, Zhang LM, Zhu B, He YT, Xiao YH. Effects of augmentation of liver regeneration recombinant plasmid on rat hepatic fibrosis. World J Gastroenterol. 2005;11:2438–2443.
- Zhang YD, Zhou J, Zhao JF, Expression, purification and bioactivity of human augmenter of liver regeneration. World J Gastroenterol. 2006;12:4401–4405.
- Taira T, Yoshimura A, Lizuka K, Expression of epidermal growth factor and its receptor in rabbits with ischaemic acute renal failure. Virchows Arch. 1996;427:583–588.
- Oka A, Tanji N, Toshino A, Miyauchi Y, Yokoyama M. Expression of growth factors after the release of ureteral obstruction in the rat kidney. Int J Urol. 1999;6:607–615.
- Landau D, Chin E, Bondy C, Expression of insulin-like growth factor binding proteins in the rat kidney: Effects of long-term diabetes. Endocrinology. 1995;136:1835–1842.
- Rabkin R, Fervenza F, Tsao T, Hepatocyte growth factor receptor in acute tubular necrosis. J Am Soc Nephrol. 2001;12:531–540.