Abstract
Introduction. This study aims to investigate gadolinium chloride (Gd) pre-treatment with/without splenectomy (Splx) in the setting of renal ischemia/reperfusion (IR) injury in rats. Materials and Methods. Under anesthesia, male Wistar albino rats with or without splenectomized (Splx) were right nephrectomized and subjected to 45 min of renal pedicle occlusion followed by 3 h of reperfusion. Gadolinium chloride (10 mg kg−1) or saline was administered 24 hours prior to ischemia via penile vein. Right nephrectomy and intravenous saline administration was performed in the control group. At the end of the reperfusion period, following decapitation, kidney samples were taken for histological examination or determination of renal malondialdehyde (MDA) and glutathione (GSH) levels and myeloperoxidase (MPO) and Na+-K+ ATPase activities. Creatinine, blood urea nitrogen (BUN), lactate dehydrogenase (LDH), TNF-α, and IL-1β were assayed in the serum samples. Results. Ischemia/reperfusion caused significant increases in the serum TNF-α, IL-1β, BUN, creatinine, AST, ALT, LDH, and tissue MDA levels and MPO activity, while either Gd pre-treatment or Splx decreased these parameters significantly. On the other hand, IR induced a decrease in the tissue GSH, and Na+-K+ ATPase activity was restored by both gadolinium and Splx. Furthermore, histopathological alterations induced by IR were also reversed. Conclusion. The extent of renal IR injury depends on the pro-inflammatory cytokine response. Gd pre-treatment decreases macrophage-derived cytokine secretion and thereby effectively limits the extent of renal IR injury in rats similar to Splx. Further studies needed to define an optimal way of decreasing macrophage-derived cytokine release due to the clinical limitations of Gd.
INTRODUCTION
Temporary cessation of renal artery flow during renal transplantation, partial nephrectomy, renal artery angioplasty, cardiopulmonary bypass, aortic bypass, shock, sepsis, and some elective urological procedures induce renal ischemia/reperfusion (IR) injury, which may result in acute renal failure and increase the procedure-related morbidity and mortality. The extent of renal IR injury depends on the amount of reactive oxygen species (ROS) formed within the ischemic tissue during the reperfusion phase and the inflammatory response reacting to ROS. Pro-inflammatory cytokines secreted from tissue macrophages such as TNF-α and IL-1β play a major role in the systemic inflammatory response to IR. These proinflammatory cytokines induce the accumulation and infiltration of neutrophils and cause IR injury within the tissue as well as remote organs.Citation[1–5] The cellular injury as a result of IR may be prevented by decreasing the inflammatory response and ROS production.Citation[6]
Regardless, the utility of minimizing the contribution of the macrophages to the development of IR injury appears to be critical. Gd, while commonly used experimentally, is an unlikely therapeutic agent, as it must be administered 24 hours prior to the initiation of ischemia. The action of Gd is reported to be specific to Kupffer cells but also affects monocytes and whole body macrophages and causes nephrogenic systemic fibrosisCitation[7–10] in repeated administration during long-term follow-ups, thus limiting its clinical applicability.Citation[11,Citation12] Splenectomy (Splx) has been reported to reduce the number of macrophages and thereby decrease the proinflammatory cytokine release in the setting of IR injury both experimentally and clinically.Citation[13,Citation14] As whole body macrophages play an important immunologic role, it is unlikely that the complete suppression of its function is clinically reasonable. However, the reduction or neutralization of the proinflammatory mediators released by whole body macrophages is clinically relevant either with decreasing the number or function of whole body macrophages. To the best of our knowledge, Gd pre-treatment with or without Splx has not been studied in the setting of renal IR in rats. This study aims to investigate the outcome of renal IR injury in rats with Gd pre-treatment with and without splenectomy.
MATERIALS AND METHODS
Animals
The study included 48 male Wistar albino rats (220–250 g). The Institutional Ethics Committee of Haydarpasa Numune Research and Training Hospital approved the experimental protocol. The study was approved by the Haydarpasa Training and Research Hospital Animal Care and Use Committee. The animals were kept in laboratory for two weeks prior to the experiment for adjustment to the environmental change, with free access ad libitum diet and water.
Surgery and Experimental Protocol
Rats were randomized into six groups eight of each. All rats were right nephrectomized.
Experimental Groups
The rats were divided into the following groups:
group 1: control (C) with bolus intravenous saline administration;
group 2: splenectomy (Splx) bolus intravenous saline administration;
group 3: ischemia reperfusion (IR) with bolus intravenous saline administration;
group 4: ischemia reperfusion with splenectomy and intravenous saline administration;
group 5: ischemia reperfusion with gadolinium (Gd); and
group 6: ischemia reperfusion with gadolinium and splenectomy
Under anesthesia (100 mg/kg ketamine and 0.75 mg/kg chlorpromazine; i.p.), male Wistar albino rats with or without splenectomized (Splx) were right nephrectomized and subjected to 45 min of left renal pedicle occlusion followed by 3 h of reperfusion.Citation[15] We used a 10 mg/kg dosage based on the reported literature demonstrating macrophage apoptosis within the range of 5 to 20 mg/kg dosage. Bolus gadolinium (10 mg/kg) or saline was administered 24 hours prior to ischemia via penile vein. We refer to the reported literature while we defined the dosage of gadolinium to administer as well as the time of administration. 24 hours of gadolinium treatment is reported to demonstrate the most significant impact on macrophages. That was our rationale to give gadolinium 24 hours prior to the experiment.Citation[11,Citation16]
Control rats were sham operated and administered saline only. The experiment took place in the setting of heating lamp and warm saline irrigation of abdominal cavity preoperatively to prevent hypothermia as well as fluid replacement. At the end of the reperfusion period, following decapitation, kidney samples were taken for histological examination or determination of renal malondialdehyde (MDA) and glutathione (GSH) levels and myeloperoxidase (MPO) and Na+-K+ ATPase activities. Creatinine, blood urea nitrogen (BUN), lactate dehydrogenase (LDH), TNF-α, and IL-1β, were assayed in the serum samples.
Biochemical Analysis
Serum creatinine, urea, AST, ALT, and LDH levels were determined spectrophotometrically using an automated analyzer.Citation[17–20] Plasma levels of TNF-α and IL-1β were quantified using specific ELISA kits according to the manufacturer's instructions (Biosource International, Nivelles, Belgium). These particular assay kits were selected because of their high degree of sensitivity, specificity, and inter- and intra-assay precision, and small amount of plasma sample required to conduct the assay.
Tissue samples were homogenized with ice-cold 150 mM KCl for the determination of malondialdehyde (MDA) and glutathione (GSH) levels. The MDA levels were assayed for products of lipid peroxidation by monitoring thiobarbituric acid reactive substance formation.Citation[21] Lipid peroxidation was expressed in terms of MDA equivalents using an extinction coefficient of 1.56 × 105 M−1cm−1, and the results were expressed as nmol MDA/g tissue. GSH measurements were performed using a modification of the Ellman procedure.Citation[22] Briefly, after centrifugation at 2000 × g for 10 min, 0.5 mL of supernatant was added to 2 mL of 0.3 mol/L Na2HPO4.2H2O solution. A 0.05 ml solution of 10 mM dithiobisnitrobenzoate (dissolved in 1% sodium citrate) was added, and the absorbance at 412 nm was measured immediately after mixing GSH levels were calculated using an extinction coefficient of 1.36 × 104 M−1cm−1. The results were expressed in ìmol GSH/g tissue.
Myeloperoxidase (MPO) is an enzyme that is found predominantly in the azurophilic granules of polymorphonuclear leukocytes (PMN). Tissue MPO activity correlates significantly with the number of PMN determined histochemically in inflamed tissues,Citation[23] and therefore, it is frequently utilized to estimate tissue PMN accumulation.
MPO activity was measured in tissues in a procedure similar to that documented by Hillegas et al.Citation[24] Tissue samples were homogenized in 50 mM potassium phosphate buffer (PB, pH. 6.0) and centrifuged at 41,400 × g (10 min); pellets were suspended in 50 mM PB containing 0.5% hexadecyltrimethylammonium bromide (HETAB). After three freeze–thaw cycles, with sonication between cycles, the samples were centrifuged at 41,400 × g for 10 min. Aliquots (0.3 mL) were added to 2.3 mL of reaction mixture containing 50 mM PB, o-dianisidine, and 20 mM H2O2 solution. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance measured at 460 nm for 3 min. MPO activity was expressed as U/g tissue.
Measurement of Na+,K+-ATPase activity is based on the measurement of inorganic phosphate released by ATP hydrolysis during incubation of homogenates with an appropriate medium. As the activity of Na+,K+-ATPase, a membrane-bound enzyme required for cellular transport, is very sensitive to free radical reactions and lipid peroxidation, reductions in this activity can indicate membrane damage indirectly. The total ATPase activity was determined in the presence of 100 mM NaCl, 5 mM KCl, 6 mM MgCl2, 0.1 mM EDTA, and 30 mM Tris HCl (pH 7.4), while the Mg2+-ATPase activity was determined in the presence of 1mM ouabain. The difference between the total and the Mg2+-ATPase activities was taken as a measure of the Na+,K+-ATPase activity.Citation[25,Citation26] The reaction was initiated with the addition of the homogenate (0.1 ml), and a 5-min preincubation period at 37°C was allowed. Following the addition of 3 mM Na2ATP and a 10-min re-incubation period, the reaction was terminated by the addition of ice-cold 6% perchloric acid. The mixture was then centrifuged at 3500 g, and Pi in the supernatant fraction was determined by the method of Fiske and Subarrow.Citation[27] The specific activity of the enzyme was expressed as nmol Pi mg−1 protein h−1. The protein concentration of the supernatant was measured by the Lowry method.Citation[28]
Histopathological Analysis
For light microscopic investigations, renal tissue specimens were fixed in 10% formaldehyde, dehydrated in alcohol series, cleared in toluene, and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E) and examined under a photomicroscope (Olympus BX51, Tokyo, Japan). All tissue sections were examined microscopically for the characterization of histopathological changes by an experienced histologist in blind fashion.
Statistical Analysis
Statistical analysis was performed using SPSS version 12. Each group consisted of eight animals. All data are expressed as the mean ± SEM. Groups of data were compared with Anova followed by Turkey's multiple comparison tests. Statistical significance was accepted as p < 0.05.
RESULTS
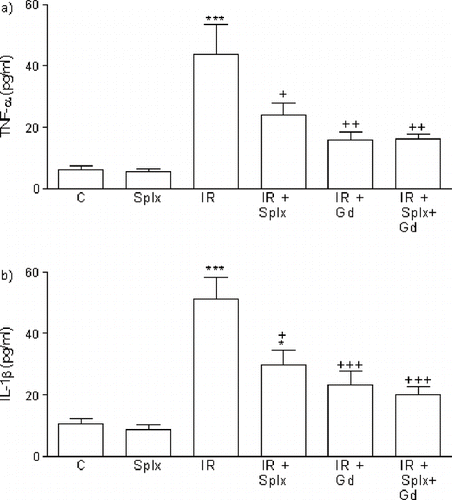
As shown in serum AST, ALT, BUN, creatinine, and LDH levels in the IR group were found to be significantly higher than those in the control rats (p < 0.01–0.001). On the other hand, either Gd pre-treatment or Splx significantly decreased these parameters (p < 0.05–0.001). Furthermore, IR caused significant increase in serum levels of proinflammatory cytokines, TNF-α, and IL-1β, which were also found significantly reduced by Gd pre-treatment or Splx (p < 0.05–0.001; see ).
Table 1 Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, and lactate dehydrogenase (LDH) levels of experimental groups
Figure 1. a) Tumor necrosis factor-α (TNF-α) and b) interleukin-1 β (IL-1 β) levels in the serum of control, splenectomy (Splx)-, and gadolinium (Gd; 10 mg/kg)-treated ischemia/reperfusion (IR) groups. Each group consists of eight animals. *p < 0.05, ***p < 0.001, compared to control group; +p < 0.05, ++p < 0.01, +++p < 0.001, compared to IR group.
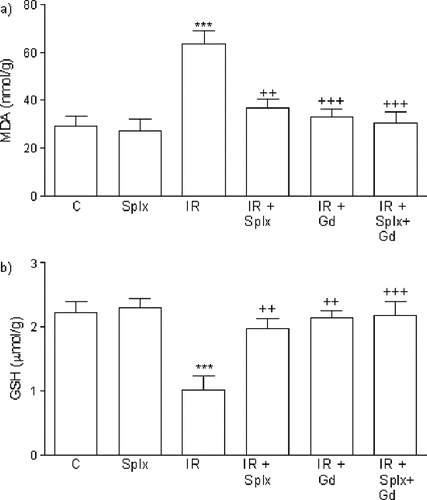
The renal tissue MDA levels in the control group were elevated by IR injury (p < 0.001); however, Gd pre-treatment significantly decreased the IR-induced elevation in renal MDA levels (p < 0.001). In the IR+Splx group, the renal MDA levels were also found significantly reduced (p < 0.01; see ). In accordance with that, IR caused a significant decrease in renal GSH level when compared to control group (p < 0.001), while both in the Gd pre-treated IR and IR+Splx groups, renal GSH content was found to be preserved (p < 0.01), not being significantly different from that of the control group (see ).
Figure 2. a) Malondialdehyde (MDA) and b) glutathione (GSH) levels in the kidney tissue of control, splenectomy (Splx)-, and gadolinium (Gd; 10 mg/kg)-treated ischemia/reperfusion (IR) groups. Each group consists of eight animals. **p < 0.01, ***p < 0.001, compared to control group; ++p < 0.01, +++p < 0.001, compared to IR group.
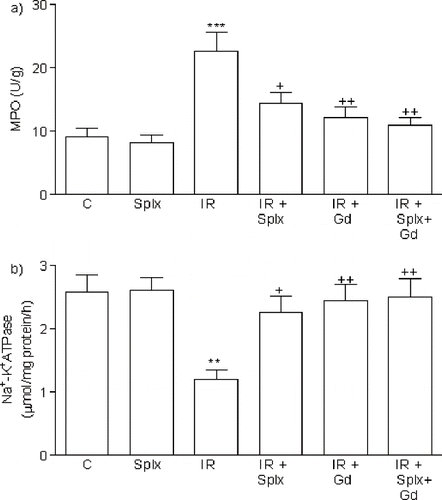
Myeloperoxidase activity, which is accepted as an indicator of neutrophil infiltration, was significantly higher in the kidney tissue of the IR group (p < 0.001) than that of the control group. On the other hand, either Gd pre-treatment or Splx significantly decreased these parameters (p < 0.05–0.001, ).
Figure 3. a) Myeloperoxidase and b) Na+-K+ ATPase activity in the kidney tissue of control, splenectomy (Splx)-, and gadolinium (Gd; 10 mg/kg)-treated ischemia/reperfusion (IR) groups. Each group consists of eight animals. **p < 0.01, ***p < 0.001, compared to control group; +p < 0.05, ++p < 0.01 compared to IR group.
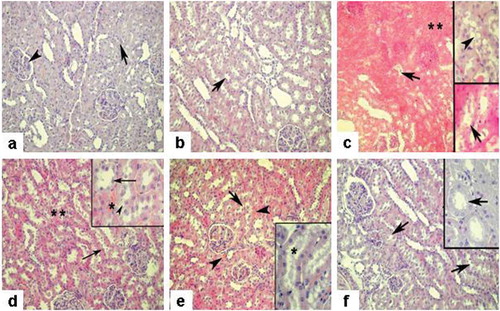
Histopathologic observation of tissues demonstrated an amelioration with the treatments of either Splx or/and Gd treatment. Glomerular and tubular structures were regular in control and Splx groups (see and ). Medullary congestion and interstitial edema besides severe vacuolization of tubular epithelial cells were detected in IR group (see ). The IR+Splx group demonstrated a regression in interstitial edema and a reduction of vacuolization of tubular epithelium, whereas partial tubular degeneration was still present (see ). IR with Gd treatment similarly demonstrated diminished tubular epithelial vacuolization (see ). Medullary congestion was reduced in the Gd-treated IR group compared to IR alone. In the IR+Gd+Splx group, tissue injury was generally reduced and tubular epithelial structures were recovered with minimally interstitial edema compared to IR alone (see ).
Figure 4. a) Sham group, regular morphology of renal glomerulus (arrowhead) and tubulus (arrow); b) Splx group, similar morphology with sham group with regular tubulus structure (arrow); c) IR group, severe medullary congestion and interstitial edema (**) besides prominent vacuolization in the cytoplasm of the tubule cells (inset, arrowhead), tubular degeneration (arrows); d) IR + Splx group, reduced edema and congestion (**), regenerated tubular cells (arrows), and partial tubular degeneration (inset-arrowhead); e) IR + Gd group, regenerated tubular cells and note the regressed profile of cellular vacuolization (inset-*), decreased and limited medullary congestion (arrowheads); f) IR + Splx+ Gd group, prominent regeneration of tissue with regular tubules (arrows) (note the regular morphology of proximal tubulus, inset-arrow). HE stain, magnification × 200, insets × 400.
DISCUSSION
It is well known that temporary cessation of renal artery flow causes structural and functional changes in kidneys. The extent of the injury related to the ischemia depends on the inflammatory response during reperfusion phase and production of ROS such as hydrogen peroxide, hydroxyl, hypochlorite, peroxynitrite, and lipid peroxyl radicals.Citation[4,Citation29] ROS production occurs within the injured tissue and also from the accumulated and infiltrated neutrophils via pro-inflammatory cytokines. Neutrophils activated by pro-inflammatory cytokines such as TNF-α and IL-1β play a major role in IR injury in the target tissue and remote organs as well.Citation[1–3] Although there is no specific treatment of IR injury, there are numerous experimental studies reporting the outcome of antioxidant treatments and inflammatory response reducing protocols in the setting of IR injury.Citation[3,Citation[30–33] We clearly demonstrated that Gd with or without Splx effectively reduced the extent of IR injury as a result of decreasing proinflammatory cytokine levels. In vivo and in vitro experimental studies have reported that Gd suppresses the reticuloendothelial system as a result of blocking macrophage migration and activation. Gd molecule is captured within macrophages by pinocytosis/phagocytosis and causes lysosomal membrane destruction, leading to an irreversible macrophage membrane destruction and the death of macrophage.Citation[34–36] On the other hand, histopathologic imagings and electron microscopic investigations demonstrated that gadolinium was present within the nucleus and conjugated with the chromatin causing apoptosis of macrophages.Citation[16] Laboratory-based experimental models detected that Gd inactivates the macrophages within the tissues of heart, lung, liver, and intestine, which decreases the adhesion molecule expression and pro-inflammatory mediator secretion leading to prevented IR injury in the given tissues.Citation[13,Citation14,Citation37,Citation38] Liver Kupffer cells and splenic monocyte/macrophage pool contribute 90% of mononuclear phagocytic system.Citation[39] Splenic macrophages are mononuclear cells responsible for both cellular and humoral immunity.
Splenectomy has been demonstrated as the method of reducing pro-inflammatory mediator secretion by reducing the number macrophages in the setting of IR.Citation[13,Citation14] In the present study, Gd administration with or without Splx prior to renal IR injury significantly reduced serum TNF-α and IL-1ß concentrations compared to renal IR alone. The studies investigating the physiopathology of the injury following reperfusion detected the major role of ROS production within the ischemic tissue, which activates the macrophages to secrete proinflammatory cytokines and trigger the inflammatory response.Citation[2–4] Accumulated neutrophils within the ischemic tissue leads to additional ROS production and secretion of protease, elastase, MPO, and proinflammatory cytokines, which alleviates the extent of cellular injury.Citation[40] In the current study, MPO activity as a marker of neutrophil activation was significantly reduced by both Splx and Gd treatment compared to IR alone. It could be estimated that Gd with or without Splx reduced pro-inflammatory cytokine secretion and thereby minimized the accumulation of neutrophils to the reperfused tissue, which prevented IR injury. Increased production of ROS causes lipid peroxidation, which is expressed with increased levels of MDA as a final production of lipid peroxidation.Citation[27] We detected significantly decreased MDA levels in IR with Gd, Splx, or both compared to IR alone, probably as a result of decreased production of ROS.Citation[41,Citation42] GSH is a strong endogenous antioxidant present in the cytoplasm that degrades hydrogen peroxide into water. In the current study, we also demonstrated that Gd with or without Splx prevented GSH decrease, as it was detected in IR alone. Na+-K+ ATPase is an enzyme responsible for Na+ and K+ exchange to maintain the intracellular homeostasis. Na+-K+ ATPase activity is related to the presence of ATP and phospholipids within the cellular membrane. Increased ROS production such as hydroxyl radicals as a result of ischemia causes lipid peroxidation.Citation[43] Decreased concentration of intracellular ATP and degraded membrane phospholipids compromises the activity of Na+-K+.
ATPase Enzyme
It has been demonstrated that in renal IR models, decreased Na+-K+ ATPase activity causes deterioration of sodium reabsorption within renal tubules and decreases GFR. We detected protection of Na+-K+ ATPase activity in IR with Gd or Splx groups compared to IR alone. Histopathological examinations revealed increased neutrophil infiltration, proximal tubule injury, nuclear pyknosis, cellular vacuolization, medullar congestion, and apoptotic cells in IR alone compared to IR with pre-treatment in the setting of renal IR injury. Acute renal failure (ARF) as a result of renal IR injury is characterized with the increase of serum creatinine and BUN. In our study, ARF was prevented in treatment groups, which was detected as normal renal function and morphology compared to IR alone. Gd with or without Splx significantly reduces the systemic markers of tissue injury such as LDH, AST, and ALT, which recalls the usefulness of these procedures (Gd or Splx) at the tissue level. ARF is a complex syndrome consisting of renal vasoconstriction, extensive renal tubular injury, insufficient glomerular filtration, and glomerular injury.Citation[44] There is no specific treatment of ARF other than supportive treatment. Pretreatment protocols aimed to reduce inflammatory response to IR injury are promising treatment alternatives.
In conclusion, tissue macrophages play a key role in IR injury by determining the extent of systemic inflammatory response via cytokine secretion. Splx causes significant reduction of tissue macrophages and has been demonstrated to prevent IR injury. Any other option of producing the same result without surgical intervention would be a promising treatment alternative of IR injury. Gd pretreatment demonstrates significant reduction of renal tissue as well as remote organ injury in the setting of renal IR. Reported literature addresses the preventive role of Gd in the setting of IR injury as decreasing the number and the function of tissue macrophages, which causes decreased cytokine secretion. To our best of our knowledge, this is the first study focused on the effect of Gd in the setting of renal IR, while most of reported studies aimed to evaluate Gd in IR injury of liver due to the findings suggesting that Gd is specific to Kupffer cells. Regardless of the limitations of clinical relevance of Gd due to both the need of administration 24 hours prior to ischemia and the side effect as systemic nephrogenic fibrosis,Citation[45] it is critical to prove the benefits of pharmacological immunomodulation by decreasing the macrophage-derived cytokine release and thereby limit the extent of renal IR injury. Further studies are needed to define a clinical applicable pharmacological method of decreasing macrophage-derived cytokine release precluding surgical intervention such as splenectomy.
ACKNOWLEDGMENTS
The authors report no conflicts of interest to disclose.
REFERENCES
- Rezende-Neto JB, Moore EE, Melo de Andrade MV, Systemic inflammatory response secondary to abdominal compartment syndrome: Stage for multiple organ failure. J Trauma. 2002;53:1121–1128.
- Pompermayer K, Souza DG, Lara GG, The ATP-sensitive potassium channel blocker glibenclamide prevents renal ischemia/reperfusion injury in rats. Kidney Int. 2005;67:1785–1796.
- Kher A, Meldrum KK, Hile KL, Aprotinin improves kidney function and decreases tubular cell apoptosis and proapoptotic signaling after renal ischemia–reperfusion. J Thorac Cardiovasc Surg. 2005;130:662–669.
- Sener G, Sehirli O, Velioglu-Ogunc A, Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol Res. 2006;54:65–71.
- Kim YK, Yoo JH, Woo JS, Effect of pentoxifylline on ischemic acute renal failure in rabbits. Ren Fail. 2001;23:757–772.
- Senga S, Onituka A, Hirose H, Protective effect of liposomal encapsulated superoxide dismutase on ischemically injured liver in the rat. Transplant Proc. 1990;22:2025–2026.
- Ito K, Ozasa H, Yoneya R, Horikava S. Splenectomy ameliorates hepatic ischemia and reperfusion injury mediated by heme oxygenase-1 induction in the rat. Liver. 2002;22:467–473.
- Savas MC, Ozguner M, Ozguner I.F, Delibas N. Splenectomy attenuates intestinal ischemia-reperfusion-induced acute lung injury. J Pediatr Surg. 2003;38:1465–1470.
- Limuro Y, Yamamoto M, Kohno H, Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats—analysis of mechanisms of lethality in endotoxemia. J Leukoc Biol. 1994;55:723–728.
- Hardonk MJ, Dijkhuis FWJ, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:296–302.
- Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387.
- Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin Wochenschr. 1965;43:174–175.
- Moss DW, Henderson AR, Kachmar JF. Enzymes. In: Tiets NW, ( ed.). Fundamentals of clinical chemistry. , Philadelphia: WB Saunders Company;1987:372–373.
- Martinek RG. A rapid ultraviolent spectrophotometric lactic dehydrogenase assay. Clin Chim Acta. 1972;40:91–99.
- Beuge JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–311.
- Beutler E. Red cell metabolism. In: Beutler E, ( ed.). A manual of biochemical methods. New York: Grune and Stratton; 1975:112–114.
- Bradley PP, Priebat DA, Christersen RD, Rothstein G. Measurement of cutaneous inflammation. Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209.
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295.
- Kim YK, Lee SH, Goldinger JM, Hong SK. Effect of ethanol on organic ion transport in rabbit kidney. Toxicol Appl Pharmacol. 1986;86:411–420.
- Reading HW, Isbir T. The role of cation activated ATPase in transmitter release from the rat iris. Q J Exp Physiol. 1980;65:105–116.
- Fiske CH, SubbaRow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Sener G, Tugtepe H, Yüksel M, Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Med Res. 2006;37:822–830.
- Alatas O, Sahin A, Colak O, Beneficial effects of allopurinol on glutathione levels and glutathione peroxidase activity in rat ischemic acute renal failure. J Int Med Res. 1996;24:33–39.
- Eschwege P, Paradis V, Conti M, Insitu detection of lipid peroxidation byproducts as markers of renal ischemia injuries in rat kidneys. J Urol. 1999;162:553–557.
- Lopez-Neblina F, Paez-Rollys AJ, Toledo-Pereyra LH. Mechanism of protection of verapamil by preventing neutrophil infiltration in the ischemic rat kidney. J Surg Res. 1996;61:469–472.
- Weight SC, Furness PN, Nicholson ML. Biphasic role for nitric oxide in experimental renal warm ischemia-reperfusion injury. Br J Surg. 1999;86:1039–1046.
- Tullis MJ, Brown S, Gewertz BL. Hepatic influence on pulmonary netrophil sequestration following intestinal ischemia-reperfusion. J Surg Res. 1996;66:143–146.
- Jahnke C, Mehrabi A, Golling M, Evaluation of microperfusion disturbances in the transplanted liver after Kupffer cell destruction using GdCl3: An experimental porcine study. Transpl Proc. 2006;38:1588–1595.
- Mizgerd JP, Molina RM, Stearns RC, Gadolinium induces macrophage apoptosis. Warner J Leukoc Biol. 1996;59:189–195.
- Lazar GJr, Lazar G, Kaszaki J, Olah J, Kiss I, Husztik E. Inhibition of anaphylactic shock by gadolinium chloride-induced Kupffer cell blockade. Agents Actions. 1994;41:97–98.
- Isao S, Koji H, Shyuji T, Kiwamu O. Gadolinium chloride reverses dimethylnitrosamine (DMN)-induced rat liver fibrosis with increased matrix metalloproteinases (MMPs) of Kupffer cells. Life Sci. 2003;72:943–959.
- Towfigh S, Heisler T, Rigberg DA, Intestinal ischemia and the gut-liver axis: An in vitro model. J Surg Res. 2000;88:160–164.
- Takaoka M, Ohkita M, Kobayashi Y, Protective effect of alpha-lipoic acid against ischaemic acute renal failure in rats. Clin Exp Pharmacol Physiol. 2002;29:189–194.
- Ross D. Glutathione, free radicals and chemotherapeutic agents. Pharmacol Ther. 1988;37:231–249.
- Herbert V, Colman N, Jayatilleke E. Effect of ethanol generated free radicals on gastric intrinsic factor and glutathione. Alcohol. 1990;7:153–157.
- Kwon TH, Frokiaer J, Han JS, Decreased abundance of major Na+ transporters in kidneys of rats with ischemia induced acute renal failure. Am J Physiol Renal Physiol. 2000;278:925–939.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460.
- Grand D, Johnsen H, Juelsrud A, Løvhaug D. Effect of gadolinium contrast agents in naïve and nephrectomized rats: Relevance to nephrogenic systemic fibrosis. Acta Radiol. 2009;50(2):156–169.
- Varani J, DaSilva M, Warner RL, Deming MO, Barron AG, Johnson KJ, Swartz RD. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol. Feb2009;44(2):74–81.
- Penfield JG. Nephrogenic systemic fibrosis and the use of gadolinium-based contrast agents. Pediatr Nephrol. Dec2008;23(12):2121–2129.
- Gazoni LM, Tribble CG, Zhao MQ, Unger EB, Farrar RA, Ellman PI, Fernandez LG, Laubach VE, Kron IL. Pulmonary macrophage inhibition and inhaled nitric oxide attenuate lung ischemia-reperfusion injury. Ann Thorac Surg. Jul2007;84(1):247–253.
- Fiser SM, Tribble CG, Long SM, Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–1075.
- Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): A late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging. Sep 242007;7(1):130–137.
- Sener G, Sehirli AO, Keyer-Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res. Mar2002;32(2):120–126.