Abstract
Patients with end stage renal disease suffer from a high incidence of infectious diseases believed to be related to their impaired immune system. To determine the antitetanus and antidiphtheria IgG antibody levels in Iranian hemodialysis patients with end stage renal disease, as well as its association with sex, age, hemoglobin, zinc serum level, serum albumin, duration of dialysis, number of dialysis per week, dialysis adequacy, erythropoietin or iron supplements such as venofer, body mass index (BMI), and underlying renal disorder, we conducted a cross-sectional study on a total of 112 hemodialysis patients with end stage renal disorder (60 male, 52 female ) and 36 healthy individuals in the control group (14 male, 22 female). The patients and the control group received no antitetanus or antidiphtheria vaccine or immunoglobulin in the year prior to the investigation. The serum antitetanus and antidiphtheria IgG antibody levels were measured using the ELISA method. We found out that only 16% of our hemodialysis patients were immune to diphtheria (19% of the control group), and this rate for tetanus was 24% (48.2% in the healthy control). Except for the hemodialysis duration, none of the mentioned factors seemed to affect immunity. We conclude that in our study, the level of the antitetanus IgG antibody (unlike the antidiphtheria IgG antibody level) is significantly different among the hemodialysis patients, the chronic hemodialysis patients, and the control group.
INTRODUCTION
The incidence of infectious disease is increased in patients with renal failure.Citation[1] This immunodeficiency status is associated with decreased immunoglobulin production, diminished interleukin-2 secretion by T lymphocytes, impaired macrophage function, and the co-stimulatory properties of antigen presenting cells.Citation[2,Citation3] Thus, hemodialysis patients represent a special population because of the above-mentioned immunodeficiency status and unique exposure, which makes it imperative that we take advantage of all potential mechanisms to prevent infectious complications in patients with renal disease.Citation[4]
This deficient immune system is related to many factors, including duration of hemodialysisCitation[5] or zinc deficiency. This is especially the case in elderly hemodialysis patients, as zinc is a catalytic, structural, and regulatory ion for enzymes, proteins, and transcription factors, and thus a key trace element in many homeostatic mechanisms of the body, including immune responses.Citation[6,Citation7] However, no definite cause has been shown to induce this immunodeficiency level yet.
As the flawful immune response of CRF patients leads to low response rates and insufficient antibody concentrations following a number of highly recommended vaccines, such as tetanus, diphtheria, and hepatitis B,Citation[8–11] chronic renal failure (CRF) patients will need revaccination much earlier than the normal population. As a result, several studies have focused on the protection of these patients by vaccination.Citation[2,Citation8]
In spite of the widespread vaccination programs against diphtheria and tetanus in the general population of Iran and other countries,Citation[12,Citation13] we still face cases of diphtheria and tetanus in human societiesCitation[14,Citation15] as well as chronic kidney disease (CKD) patients, in whom we have previously shown a significant difference in the antitetanus protection rate (52.8%) compared to that of healthy individuals (74.3%).Citation[5]
In addition, it has been demonstrated that protection against diphtheria and the immune response to Td vaccine in hemodialysis (HD) patients is poor (i.e., a seroconversion rate of 37%).Citation[16,Citation17] It is also noteworthy to mention that the response to tetanus and diphtheria vaccinations is highly related (p < 0.04).Citation[1]
Although several studies have focused on the protection of HD patients against infections by vaccination, no guidelines are yet available for preventive care in patients with CKD, including the use of vaccines such as the Td booster vaccine.Citation[16] This study was conducted to illustrate the state of protection against tetanus and diphtheria in HD patients and to determine any attributing factors.
MATERIALS AND METHODS
Patients and Control Group Selection
This study was conducted on a total of 112 patients under hemodialysis due to CRF in the Hemodialysis Center of Shiraz University of Medical Science in 2006–2007. Their mean age was 54.5 ± 14 (60 male, 52 female). On the other hand, 36 individuals (14 male, 22 female) with a mean age of 53.9 ± 9.9 were selected from our patients' relatives to form the control group. Neither the patients nor the control group received any immunosuppressive medications prior to our study. The control group had no renal disorders and was matched by the patients group based on age and sex. Although we faced an unreliable past vaccination history in our patients, we managed to exclude patients who received any antitetanus toxoid vaccine or immunoglobin in the previous year by referring to their medical records. As immunization against diphtheria, tetanus has been applied since 1950 in IranCitation[13]; we expected that our patients, who are greater than 66 years old, had received the Td vaccine at least once in their lifetime.
Data Collection and Detection of Anti-tetanus and Anti-diphtheria Antibodies
Data including sex, age, hemoglobin, zinc serum level, serum albumin, duration of dialysis, number of dialysis per week, dialysis adequacy, erythropoietin or iron supplements such as venofer, body mass index (BMI), and underlying renal disorder were obtained from all the hemodialysis patients medical records.
The serum zinc level of the patients was checked with the atomic absorption method.
The levels of antitetanus toxoid IgG and antidiphtheria IgG were measured by enzyme linked immunosorbent assay (ELISA) method on serum extracted from the blood samples of the CRF patients before the start of hemodialysis. Sera were separated by centrifuge (10 min, 206 × g, 4°C) and stored at -20°C until analysis. Antibody levels were then measured by commercial ELISA kits (IBL-Hamburg GmbH, Hamburg, Germany). Optical density was measured at 450 nm using ELISA reader (Anthos Labtec Instruments, Austria). In accordance with the EPI Program of the WHO, the assay cut-off for the protective level of the antitetanus toxoid IgG antibody was set at 0.1 international units per ml (IU/mL), and 1 IU/mL for the diphtheria IgG antibody due to the manufacture's advice. We considered the concentrations above the assay cut-offs to be seroprotective.Citation[1,Citation18]
Ethics and Statistical Analysis
The study was approved by the Ethical Committee in Shiraz University of Medicine.
Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, Illinois, USA). Statistical differences of various clinical and laboratory parameters between groups were evaluated by chi-square or Mann-Whitney U, and to compare the mean of two groups, the two independent sample t-tests were used. p values of less than 0.05 were considered significant.
RESULTS
Anti-tetanus IgG and Anti-diphtheria IgG Levels of Patients vs. Control Group
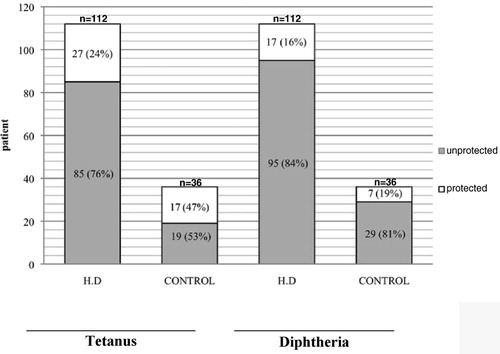
The mean serum anti tetanus IgG level of hemodialysis patients was 0.21 ± 0.46 IU/mL, and the mean antidiphtheria IgG level was 0.38 ± 0.37. Comparing with the control group′s mean anti-tetanus IgG of 0.573 ± 1.13 IU/mL and mean anti-diphtheria IgG level of 0.43 ± 0.44, it seems that, unlike the significant difference in anti-tetanus IgG between the control and patient groups (p = 0.08), there is no statistically meaningful difference in anti-diphtheria IgG between these two groups (p = 0.48).
As shown in , only 16% of our HD patients were immune to diphtheria, and for tetanus this rate was 24%. shows some differences between the control and the patient group.
Table 1 Comparison of age, gender, and tetanus and diphtheria antibody between patients and control groups
Categorization of Patients Due to Their Antibody Levels
We divided the patients according to their anti-tetanus IgG level into three groups: the first group with IgG < 0.1 IU/mL, who were not protected and needed basic immunization by tetanus booster vaccine (95 patients, 19 control individuals); the second group with 0.1 < IgG < 1 IU/mL, to be controlled in 1–2 years (17 patients, 11 control individuals); and the third group with 1 < IgG < 5 IU/mL, who were required to be controlled over a 2–4-year period (10 patients, 6 control individuals).
It is also possible to categorize the patients due to their anti-diphtheria IgG level: the first group with IgG < 0.1 IU/mL who were not protected and needed basic immunization (35 patients, 13 control individuals); the second group with 0.1 < IgG < 1 IU/mL, who were required to receive booster vaccinations (60 patients, 16 control individuals); the third group with 1 < IgG < 1.5 IU/mL, who were required to be boosted in 5 years (17 patients, 7 control individuals); the fourth group with 1.5 < IgG < 2 IU/mL to be boosted in 7 years; and the fifth group 2 < IgG IU/mL to be boosted in 10 years.
Thus, as is illustrated in , most of our patients were included in the category that needs booster vaccinations for both diphtheria and tetanus.
Patient's Characteristics
The patients group consisted of 112 CRF patients under HD at the time. illustrates some of their details.
Table 2 Details of the patients group
Among our available data, including sex, age, hemoglobin, serum zinc level, albumin, duration of dialysis, number of dialysis per week, dialysis adequacy, erythropoietin or iron supplement such as venofer, body mass index (BMI), and underlying renal disorders, only the duration of hemodialysis seems to have a significant influence on the anti-tetanus IgG level in hemodialysis patients (p = 0.05).
On the other hand, we found no significant correlation between the anti-diphtheria antibody and the other factors except the anti-tetanus IgG level (p = 0.001).
DISCUSSION
Diphtheria and tetanus are two preventable diseases due to the introduction of Td and DTP vaccines. Although the vaccination against tetanus has led to a significant decrease in the outbreak of the disease after infection with Clostridium tetani,Citation[1] recent epidemiologic studies indicate that the number of insufficiently protected individuals has increased, especially in the elderly.Citation[18,Citation19] These studies also indicate a low immunity to diphtheria in adults in industrialized countries based on epidemics in Russia and the Ukraine.Citation[20]
Although the state of immunodeficiency in CRF patients leads to a more fatal prognosis in the case of infectious diseases,Citation[21] few studies have focused on immunization against tetanus and diphtheria in CRF patients, especially in Iran; for example, currently, there is not any reliable studies in literature to show the prevalence of tetanus or diphtheria infection or immunity against them in Iranian CRF patients.
In the current study, 24% (27/112) of patients and 48.2% (17/36) of the healthy control group were protected against tetanus. In a study conducted by Kruger et al., out of 71 patients on chronic hemodialysis, only 31 (44%) were protected against tetanus, while the rate was 89% (8/9) in his healthy control group.Citation[1] In another study from Guerin et al., the immunity rate against tetanus in HD patients was 39.4%.Citation[22]
Moreover, in our patients, only 16% (17/112 patients) were protected against diphtheria. This rate for the control group was 19% (7/36 individuals). These results are comparably lower than the rate of protection against diphtheria in Western healthy Europeans, which is 31%Citation[6] and 22% in 228 HD cases of Kruger.Citation[20]
We also found a lower protection rate against diphtheria in both hemodialysis and control groups, as compared with Karakus et al.Citation[23]
Previously, the Kruger study showed that during a five-year period, antibody persistence against the tetanus toxoid is better than that against the diphtheria toxoid.Citation[2] It is noteworthy to mention that due to our results, not only is the rate of protection against diphtheria lower than the rate of protection against tetanus in both the control and HD patients, but also, unlike the serum anti-tetanus IgG level (p = 0.008), there was no meaningful difference in anti-diphtheria IgG between the control and the patients groups (p = 0.48). Thus, in general, our population seems to be more susceptible to diphtheria than tetanus. This lower protection rate was also showed in pregnant ladies. The true cause of this higher rate of protection to tetanus is not clear but most probably is due to tetanus toxoid vaccination following injuries.Citation[24]
Despite this susceptibility, recent reports have shown that the incidence rate of tetanus infection has a significant sharp decline mainly due to the advancement of the knowledge of the general population and improvement of medical care facilities over the last several years.Citation[25]
Among the data collected from patients medical records, only duration of hemodialysis seems to have a negative effect on the anti-tetanus IgG level of hemodialysis patients (p = 0.051); however, this factor does not influence the diphtheria IgG level (p = 0.76).
The only factor influencing the anti-diphtheria IgG was the anti-tetanus IgG level (p = 0.001). This finding supported the previous findings that there is a strong correlation between immune response to tetanus and diphtheria toxoid, both of which are T-cell-dependent (p < 0.04).Citation[1]
Regarding serum zinc level, it is worth mentioning that the anti-diphtheria IgG and anti-tetanus IgG level in general do not correlate with the zinc serum level (p = 0.63 and 0.42), which does not support the study done by Kreft et al. This suggests a correlation between the impaired immune response to diphtheria vaccination in elderly chronic hemodialysis patients with zinc deficiency.Citation[16]
Also, there was no significant difference in either the anti-tetanus IgG (p = 0.285) or the anti-diphtheria IgG (p = 0.487) between zinc-deficient (zinc < 70, 25.9% of the hemodialysis patients) and zinc-sufficient patients (74% of the hemodialysis patients).
Regarding the age factor, Guerin et al. previously showed that age significantly impaired the immune response to tetanus booster vaccine in hemodialysis patients,Citation[22] but in this current study, age did not have any correlation with antitetanus (p = 0.52) or antidiphtheria antibody level (p = 0.206).
To conclude, it seems that most of our hemodialysis patients need booster tetanus and diphtheria vaccinations to increase their immunity against these diseases, as most of them are not protected against tetanus and diphtheria.
ACKNOWLEDGMENT
This study was partially financed by the Shiraz Cancer Research Centre, and supported by a grant from the Uronephrology Research Centre of Shiraz University of Medical Sciences, number 86_3893. The authors are grateful to the patients and their family members who participated in this study. We are also indebted to the personnel of Shiraz Faghihi Hospital and Hemodialysis Center for their assistance.
REFERENCES
- Kruger S, Seyfarth M, Sack K, Kreft B. Defective immune response to tetanus toxoid in hemodialysis patient and its association with diphtheria accination. Vaccine. 1999;17:1145–1150.
- Kruger S, Muller Steinhardt M, Kirchner H, Kreft B. A five-year follow-up on antibody response after diphtheria and tetanus vaccination in hemodialysis patients. Am J Kidney Dis. 2001;38:1264–1270.
- Grindt M, Kohler H, Schieldhelm-Weik E, Meyer zum Buschenfelde KH, Fliescher B. T cell activation defect in hemodialysis patients: Evidence for a role of B7/CD28 pathway. Kidney Int. 1993;44:359–365.
- Dinits-Pensy M, Forrest GN, Cross AS, Hise MK. The use of vaccine in adult patients with renal disease. Am J Kidney Dis. 2005;46:997–1011.
- Sagheb MM, Sajjadi S, Sajjady G. Antitetanus toxoid antibody titre of chronic hemodialysis patients in Iran. Iran J Immunol. 2008;5:44–50.
- Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85(3):837–844.
- Ferencík M, Ebringer L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol (Praha). 2003;48(3):417–426.
- Kleinkneth C, Somerville W, Grey V, Gaiffe M, Sahyoun S, Broyer M. Serum antibodies before and after immunization in hemodialysis children. Proc Eur Dial Transplant Assoc. 1977;14:209–214.
- Trollfors B, Knutson N, Taranger J, Mark A, Bergfors E, Sundh V, Lagergard T. Diphtheria, tetanus and pertussis antibodies in 10-year-old children before and after a booster dose of three toxoids: Implications for the timing of a booster dose. Eur J Pediatr. 2006;165:14–18.
- Storsaeter J, Wolter J. Is there a need for a new generation of vaccines against pertussis?. Expert Opin Emerg Drugs. 2006;11:195–205.
- Skowera A, de Jong EC, Schuitemaker JH, Allen JS, Wessely SC, Griffiths G, Analysis of anthrax and plague biowarfare vaccine interactions with human monocyte-derived dendritic cells. J Immunol. 2005;175:7235–7243.
- Mirchamcy H. Study on diphtheria, tetanus combined immunization in children in some elementary school of Tehran. Arch Inst Razi. 1960;12:9–18.
- Zarei S, Jeddi-Tehrani M, Akhondi MM, Zeraati H, Kheirkhah T, Ghazanfari M, Immunogenicity of a triple diphtheria tetanus whole cell pertussis vaccine in Iranian preschool children. Iran J Immunol. 2007;4:101–109.
- Immunization summary. UNICEF. 2006. Available from: http://www.unicef.org/publications/files/immunization_summary_2006.pdf.
- Immunization summary. WHO. 2006. Available from: http://www.who.int/vaccines_documents/global_summary.pdf [].
- Kreft B, Fischer A, Kruger S, Sack K, Kirchner H, Rink L. The impaired immune response to diphtheria vaccination in elderly chronic hemodialysis patients is related to zinc deficiency. Biogerontology. 2000;1:61–66.
- Fushshaber A, Kahnemand O, Keath B, Lutticken R, Michalk D, Querfeld A. Pneumococcal vaccine in children and young adults with chronic renal disease. Nephrology Dialysis Transplantation. 1996;468–473.
- Klouche M, Gorg S, Wilhelm D, Kirchner H. Sex- and age-dependent gaps in tetanus immunization. Dtsch Med Wochenschr. 1994;119:827–832 [in German].
- Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761–766.
- Kreft B, Klouche M, Kreft R, Kirchner F, Sack K. Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney Int. Jul1997;52(1):212–216.
- Girndt M, Pietsch M, Kohler H. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am J Kidney Dis. 1995;26:454–460.
- Guerin A, Buisson Y, Nutini MT, Saliou P, London G, Marchais S. Response to vaccination against tetanus in chronic haemodialysed patients. Nephrol Dial Transplant. 1992;7:323–326.
- Karakus R, Aral AL, Kanat DO, Hizel K, Caglar K, Sindel S, Yetkin I, Aybay C. Determinants of protection against diphtheria in adult hemodialysis patients. Ren Fail. 2007;297:829–834.
- Saffar MJ, Khalilian AR, Ajami A, Saffar H, Qaheri A. Seroimmunity to diphtheria and tetanus among mother-infant pairs: The role of maternal immunity in infant immune response to diphtheria-tetanus vaccination. Swiss Med Wkly. 2008;138(17–18):256–260.
- Beheshti S, Khajehdehi A, Rezaian G, Khajehdehi P. Current status of tetanus in Iran. Arch Iranian Med. 2002;5(4):216–218.