Abstract
Background. Interleukin-12 (IL12) participates in the pathophysiology of various experimental types of progressive glomerulonephritis, but its role in acute mesangial glomerulonephritis (AMG) induced by habu snake venom (HSV) has not been determined. This study aims to evaluate the effect of the absence of IL12 on AMG induced by HSV. Methods. AMG was induced in IL12 knockout (IL12–/–) and C57Bl/6 (IL12+/+) mice by a single i.v. administration of HSV. Vehicle was used in control animals. Mice were studied after 3, 7, and 14 days (D3, D7, and D14). Results. After treatment with HSV, IL12+/+ and –/– mice developed focal glomerular lesions, but groups of both lineages showed no statistical difference concerning albuminuria, serum creatinine, histopathology, number of cells by glomerular tuft, and glomerular tuft area. Compared to IL12+/+ mice, IL12–/– mice showed lower scores of glomerular desmin expression on D7 [1.55 (1.32; 1.65) vs. 1.12 (1.07; 1.22); p < 0.01] and D14 [1.60 (1.55; 1.75) vs. 1.20 (1.15; 1.20); p < 0.001], respectively, and lower scores of glomerular α-SMA expression on D14 [0.30 (0.21; 0.38) vs. 0.16 (0.26; 0.36); p < 0.001], respectively. Conclusion. The absence of IL12 reduced the activity of mesangial cells, but did not modify the course of HSV-induced AMG in mice.
INTRODUCTION
Interleukin-12 (IL12) is a heterodimeric cytokine consisting of two covalently linked protein subunits denoted p35 and p40. These subunits are coded by different genes, and the expression of both genes is needed to produce biologically active IL12.Citation[1,Citation2] IL12 is mainly produced by antigen-presenting cells such as dendritic cells, macrophages, monocytes, and B lymphocytes. Its main effect appears to be the regulation of T cells and natural killer (NK) cells related to cytotoxicity.Citation[3] IL12 induces differentiation of T cells into type 1 T helper cells (Th1) phenotype by the production of IL2 and interferon-γ. In addition, neutrophils and NK cells produce platelet-activating factor (PAF) and free radicals when stimulated by IL12. This cytokine also has the property of inducing chemotaxis of neutrophils and NK cells.Citation[4]
Studies have demonstrated that IL12 participates in the pathophysiology of various experimental types of glomerulonephritis, and the absence of IL12 prevented the development of anti-glomerular basement membrane glomerulonephritis.Citation[5] IL12 produced by intrinsic renal cells (non-immune cells) also contributes to inflammatory renal injury.Citation[6]
Intravenous injection of habu snake venom (HSV) provokes acute focal proliferative mesangial glomerulonephritis in rats,Citation[7,Citation8] rabbits,Citation[9] and mice.Citation[10] The course of the morphological changes has been defined, and the damage usually heals spontaneously between two and four weeks.Citation[8,Citation11] The participation of IL12 in this experimental model of acute mesangial glomerulonephritis has not been investigated. The aim of the present study was to determine whether the absence of IL12 interferes with the course of HSV-induced glomerulonephritis in mice.
MATERIALS AND METHODS
Mice and Treatments
The study was approved by the Biosafety Committee of the School of Medicine of Ribeirão Preto, University of São Paulo, and all animal studies were performed in accord with its guidelines for the care and use of laboratory animals. Male C57Bl/6 IL12p40–/– or +/+ mice (Jackson Laboratory, Bar Harbor, Maine, USA) aged 8 to 10 weeks were injected intravenously with either 2.0 mg/kg body weight of HSV (Trimeresurus flavoviridis, Sigma-Aldrich, St. Louis, Missouri, USA) or an equal volume of 0.15 M NaCl solution. At 3, 7, and 14 days, after being housed for 15 hours in metabolic cages for urine sample collection, groups of 8 to 10 mice were anesthetized for blood sample collection by cardiac puncture, and the kidneys were removed. Samples from the right kidney were fixed either in alcoholic Bouin or methyl Carnoy and embedded in paraffin.
The absence of IL12 in IL12–/– mice, as well as its presence in IL12+/+ mice, was insured by genotyping the C57BL/6 IL12+/+ and IL12–/– mice.
Biochemical Analysis
Renal function was evaluated by serum creatinine levels.
Kidney Histology and Morphometry
Light microscopic analysis of the renal cortex was performed on 3 μm-thick paraffin sections of kidney samples fixed in Bouin's solution, and morphometry was performed on Masson's trichrome slides. Twenty consecutive glomerular cross-sections from each stained histologic section were measured for glomerular tuft area, and the number of nuclei/glomerular tuft was counted with a light camera connected to an image analyzer (KS-300; Kontron Electronik, Munich, Germany).
Immunohistochemical Analysis
Immunohistochemistry was performed on methyl Carnoy-fixed tissue sections. Sections were deparaffinized in xylene and rehydrated in a decreasing ethanol series. Endogenous peroxidase was quenched with H2O2 for 10 minutes. Sections were incubated with primary antibody diluted in 1% BSA/phosphate-buffered saline (PBS), followed by a biotinylated antibody (Vector Laboratories, Burlingame, California, USA) and ABC-Elite reagent (Vector Laboratories). The reaction product was visualized with 3.3’diaminobenzidine and by nickel chloride enhancement. The slides were counterstained with methyl green.
The antibodies used in these studies were a mouse monoclonal IgG2a antibody (clone 1A4) to an NH2-terminal synthetic decapeptide of α-smooth muscle actin (α-SMA; Dako, Glostrup, Denmark), mouse monoclonal IgG antibody anti-human desmin (clone D33, cat. Nº M0760, Dako Corporation, Denmark), a mouse IgG monoclonal antibody anti-human PCNA (clone PC10; Oncogene Research Products, San Diego, California, USA), and a rat anti-mouse MAC-2 IgG monoclonal antibody to detect glomerular macrophages (Cedarlane, Hornby, Ontario, Canada). For all biopsies, negative controls consisted of substitution of the primary antibody with equivalent concentrations of an irrelevant antibody from the same species.
The number of PCNA and MAC-2-positive cells/glomerular tuft in each section was calculated by counting the number of positive cells in 20 consecutive glomerular tufts. Glomerular expression of α-SMA was scored semiquantitatively using five grades as follows: 0 = absent staining or less than 5% of the area stained; 1 = 5–25%; 2 = 25–50%; 3 = 50–75%, and 4 = >75%.Citation[12] Each score reflects mainly changes in the extent rather than the intensity of staining and depends on the percentage of the glomerular tuft area showing positive staining.
Statistical Analysis
Data are expressed as median and first and third quartiles. Statistical analyses were performed using the Mann-Whitney U test, and p values < 0.05 were considered statistically significant.
RESULTS
Albuminuria was undectectable in all IL12+/+ e IL12–/– control and HSV-treated mice. The HSV-treated IL12+/+ and IL12–/– mice did not show a significant difference in serum creatinine at the 3, 7, and 14-day timepoints compared to their respective control IL12+/+ and IL12–/– mice.
IL12+/+ and IL12–/– control mice showed normal pattern of renal tissue. After three days of HSV treatment, IL12+/+ and IL12–/– mice presented focal intraglomerular aneurysms as the predominant lesions. On the seventh day of HSV treatment, IL12+/+ and IL12–/– mice presented segmental and focal expansion of the extracellular matrix associated with hypercellularity and focal areas of interstitial expansion and hypercellularity. On the 14th day, most of the renal parenchyma showed a normal pattern.
Concerning glomerular tuft area and the number of nuclei/glomerular tuft, there was no significant difference between IL12+/+ and IL12–/– HSV-treated mice at the three timepoints compared to their respective IL12+/+ and IL12–/– control mice.
The renal expression of α-SMA is illustrated in –1D, and the graphic depicts the α-SMA score (see ). The score to the glomerular expression of α-SMA was significantly higher (p < 0.05) to IL12+/+ and IL12–/– HSV-treated groups at the three timepoints compared to their respective control groups. When HSV-treated mice were compared at the same time point, the IL12+/+ group presented a higher α-SMA score (p < 0.01) than the IL12–/– group only on D14.
Figure 1. Representative sections of glomerular immunostaining for α-smooth muscle actin (A–D) and desmin (F–I) in IL12+/+ (A, F) and IL12–/– (B, G) control mice and IL12+/+ (C, H) and IL12–/– (D, I) HSV-treated mice. Graphs depict the score of glomerular expression of (E) α-smooth muscle actin and (J) desmin, and horizontal bars mean median (objective X40).
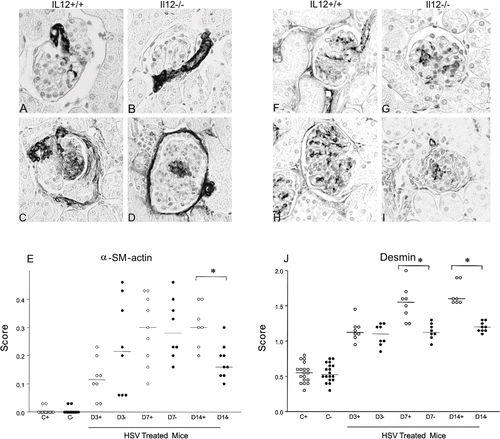
The renal expression of desmin is illustrated in –1I, and the graphic depicts the desmin score (see ). Desmin expression was increased in both IL12+/+ and IL12–/– mice treated with HSV at the three timepoints studied compared to their respective control groups, and was higher in IL12+/+ HSV-treated mice than in IL12–/– HSV-treated mice on D7 (p < 0.01) and D14 (p < 0.001).
The renal expression of PCNA is illustrated in –2D, and the graphic depicts the PCNA score (see ). The number of PCNA-positive cells/glomerular tuft was increased in IL12+/+ and IL12–/– mice at the three timepoints compared to their respective controls, but there was no statistically significant difference between HSV-treated IL12+/+ mice and IL12–/– mice.
Figure 2. Representative sections of glomerular immunostaining for PCNA (A–D) and for Mac-2 (F–I) in IL12+/+ (A, F) and IL12–/– (B, G) control mice, and in IL12+/+ (C, H) and IL12–/– (D, I) habu snake venom-treated mice. Graphs depict the number of PCNA (E) and Mac-2 (J) positive cells/glomerular tuft, and horizontal bars mean median (objective X40).
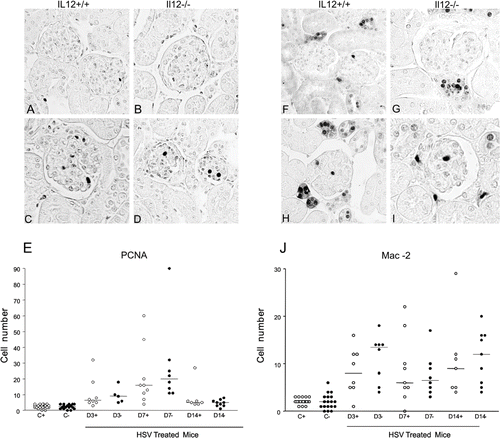
The renal expression of MAC-2 is illustrated in –2I, and the graphic depicts the MAC-2 score (see ). There was an increase in MAC-2-positive cells/glomerular tuft in IL12+/+ and IL12–/– mice at the three timepoints studied compared to their respective controls. However, there was no statistically significant difference between HSV-treated IL12+/+ mice and IL12–/– mice.
DISCUSSION
In the present study, we evaluated the participation of IL12 in the course of acute mesangial glomerulonephritis induced in mice by habu snake venom. In this study, the absence of IL12 did not produce relevant changes in the course or expression of some cellular mediators involved in this mesangial glomerulonephritis.
The absence of IL12 reducing glomerular injury has been demonstrated in experimental models such as crescent glomerulonephritis,Citation[5,Citation6,Citation13,Citation14] lupus glomerulonephritis,Citation[15] and IgA nephropathy.Citation[16]
Increased glomerular cell proliferation in HSV-induced mesangial glomerulonephritis has been previously described. As previously reported, the expression of PCNA-positive glomerular cells reached a maximum peak at day 3, followed by a rapid reduction.Citation[17] The phenotypic identification of those proliferating cells showed they were almost exclusively of mesangial origin and presented labeling for Thy-1 antigen during the first 48 hours, followed by labeling for α-SMA at 72 hours and thereafter.Citation[18,Citation19] In the present study, there was an expressive glomerular increase in the number of PCNA-positive cells in IL12+/+ and IL12–/– mice with HSV-induced glomerulonephritis, but the absence of IL12 did not modify the number of PCNA-positive cells.
Several studies demonstrated the participation of macrophages in HSV-induced glomerulonephritis starting within the first 24 hours, becoming more intense during the subsequent 72 hours,Citation18-22 and persisting for two weeks.Citation[22] In our study, there was an increased glomerular expression of macrophages in animals with HSV-induced glomerulonephritis, but the absence of IL12 did not reduce the glomerular infiltration by these cells. Experimental lupus nephritis in IL12-deficient mice (IL12p40–/– mice) presented attenuation of the histological lesions and of the amount of glomerular macrophages and T cells (CD4 and CD8).Citation[15] IL12 production by macrophages is regulated in a different manner in strains predisposed to autoimmune diseases according to the nature of the disease: their production is elevated in non-obese mice predisposed to diabetes (NOD)Citation[23] and is reduced in the lupus-prone NZB/W and MRL/+ strains.Citation[24] It has been demonstrated that these differences in IL12 production in these strains are linked to changes in the metabolism of nuclear factor-κB (NF-κB).Citation[25,Citation26] More specifically, binding of the c-Rel/p50 heterodimer predominates in macrophage extracts of NOD mice, while binding of the inhibitory p50 homodimer predominates in macrophage extracts of lupus-prone mice.Citation[25] We do not know if the different characteristics of IL12 regulation in different strains may interfere with the course of HSV-induced glomerulonephritis because this aspect was not evaluated in the present study.
Several reports showed increased glomerular expression of α-SMA in HSV-induced glomerulonephritis in rats,Citation[18-22,Citation27] especially 72 hours after the HSV injection.Citation[18,Citation20] The α-SMA-positive cells are located in proliferative lesions, but they are not present in areas bordering the proliferation, although desmin-positive and Thy-1 cells are present there.Citation[19] The region of intron 1 that plays a role in α-SMA transcription in mesangial cells activated in vivo in this experimental model of glomerulonephritis has also been described.Citation[27] In the present study, the absence of IL12 reduced the glomerular expression of α-SMA only on D14 compared to HSV-treated IL12+/+ mice. This effect was expected to occur at some intensity because mesangial cells produce mRNA and IL12 protein, express the β1 chain receptor of IL12, and respond to direct stimulation by producing platelet activating factor and free radicals.Citation[28] In addition, IL12 seems to participate in the reorganization of the cytoskeleton and in the change of cell shape.Citation[28]
Desmin is constitutively expressed in mesangial cells. As also observed for α-SMA, in our study there was an increased glomerular expression of desmin, similar to described in another study.Citation[19] Under normal conditions, desmin is expressed only by mesangial cells, but it is also expressed in podocytes in proteinuric glomerulonephritis.Citation[29] Because the glomerulonephritis evaluated here was not associated with proteinuria, the increased expression of desmin can be attributed only to mesangial cells. However, IL12–/– HSV-treated mice showed a lower glomerular expression of desmin than IL12+/+, suggesting that the absence of IL12 reduced the activity of mesangial cells but did not modify the course of glomerulonephritis.
In conclusion, the absence of IL12 seems to reduce the activity of mesangial cells, but it did not modify the course of HSV-induced acute mesangial glomerulonephritis in mice. These results suggest that IL12 may participate in the pathophysiology of the HSV-induced glomerulonephritis, but its absence is not critical to interfere with the course of this acute and self-limited mesangial glomerulonephritis.
ACKNOWLEDGMENTS
We wish to express our appreciation to Erika P. Delloiagono Gual and to Adriana L. Gonçalves de Almeida for expert technical assistance. Research supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant 477142/2003-6), by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and by Fundação de Ensino, Pesquisa e Assistência (FAEPA) do HCFMRP-USP. Roberto S. Costa, Terezila M. Coimbra and Márcio Dantas were recipients of CNPq, DF, Brazil, fellowships.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: Sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10(4):742–780.
- Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W, Familletti PC, Gately MK, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148(11):3433–3440.
- Trinchieri G. Interleukin-12: A cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84(12):4008–4027.
- Bussolati B, Mariano F, Cignetti A, Guarini A, Cambi V, Foa R, Piccoli G, Camussi G. Platelet-activating factor synthesized by IL-12-stimulated polymorphonuclear neutrophils and NK cells mediates chemotaxis. J Immunol. 1998;161(3):1493–1500.
- Le Hir M, Ryffel B, Schatzmann U. IL-12-dependent, INF-gamma-independent experimental glomerulonephritis. Kidney Blood Press Res. 2001;24(1):27–32.
- Timoshanko JR, Kitching AR, Holdsworth SR, Tipping PG. Interleukin-12 from intrinsic cells is an effector of renal injury in crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12(3):464–471.
- Cattell V. Focal mesangial proliferative glomerulonephritis in the rat caused by habu snake venom: The role of platelets. Br J Exp Pathol. 1979;60(2):201–208.
- Cattell V, Bradfield JW. Focal mesangial proliferative glomerulonephritis in the rat caused by habu snake venom: A morphologic study. Am J Pathol. 1977;87(3):511–524.
- Morita T, Kihara I, Oite T, Yamamoto T, Suzuki Y. Mesangiolysis: Sequential ultrastructural study of habu venom-induced glomerular lesions. Lab Invest. 1978;38(1):94–102.
- Eitner F, Westerhuis R, Burg M, Weinhold B, Grone HJ, Ostendorf T, Ruther U, Koch KM, Rees AJ, Floege J. Role of interleukin-6 in mediating mesangial cell proliferation and matrix production in vivo. Kidney Int. 1997;51(1):69–78.
- Bradfield JW, Cattell V, Smith J. The mesangial cell in glomerulonephritis, II: Mesangial proliferation caused by habu snake venom in the rat. Lab Invest. 1977;36(5):487–492.
- Kliem V, Johnson RJ, Alpers CE, Yoshimura A, Couser WG, Koch KM, Floege J. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49(3):666–678.
- Kitching AR, Tipping PG, Holdsworth SR. IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol. 1999;29(1):1–10.
- Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR, O'Sullivan KM, Tipping PG, Takeda K, Akira S, IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol. 2005;16(7):2023–2033.
- Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170(7):3915–3925.
- Nogaki F, Muso E, Kobayashi I, Kusano H, Shirakawa K, Kamata T, Oyama A, Ono T, Miyawaki S, Yoshida H, Interleukin 12 induces crescentic glomerular lesions in a high IgA strain of ddY mice, independently of changes in IgA deposition. Nephrol Dial Transplant. 2000;15(8):1146–1154.
- Kitamura H, Sugisaki Y, Yamanaka N. Endothelial regeneration during the repair process following habu-snake venom induced glomerular injury. Virchows Arch. 1995;427(2):195–204.
- Barnes JL, Abboud HE. Temporal expression of autocrine growth factors corresponds to morphological features of mesangial proliferation in habu snake venom-induced glomerulonephritis. Am J Pathol. 1993;143(5):1366–1376.
- Barnes JL, Hevey KA, Hastings RR, Bocanegra RA. Mesangial cell migration precedes proliferation in habu snake venom-induced glomerular injury. Lab Invest. 1994;70(4):460–467.
- Barnes JL, Hastings RR, De la Garza MA. Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol. 1994;145(3):585–597.
- Barnes JL, Mitchell RJ, Kanalas JJ, Barnes VL. Differential expression of thrombospondin and cellular fibronectin during remodeling in proliferative glomerulonephritis. J Histochem Cytochem. 1999;47(4):533–544.
- Barnes JL, Torres ES, Mitchell RJ, Peters JH. Expression of alternatively spliced fibronectin variants during remodeling in proliferative glomerulonephritis. Am J Pathol. 1995;147(5):1361–1371.
- Alleva DG, Pavlovich RP, Grant C, Kaser SB, Beller DI. Aberrant macrophage cytokine production is a conserved feature among autoimmune-prone mouse strains: Elevated interleukin (IL)-12 and an imbalance in tumor necrosis factor-alpha and IL-10 define a unique cytokine profile in macrophages from young nonobese diabetic mice. Diabetes. 2000;49(7):1106–1115.
- Alleva DG, Kaser SB, Beller DI. Intrinsic defects in macrophage IL-12 production associated with immune dysfunction in the MRL/++ and New Zealand Black/White F1 lupus-prone mice and the Leishmania major-susceptible BALB/c strain. J Immunol. 1998;161(12):6878–6884.
- Liu J, Beller D. Aberrant production of IL-12 by macrophages from several autoimmune-prone mouse strains is characterized by intrinsic and unique patterns of NF-kappa B expression and binding to the IL-12 p40 promoter. J Immunol. 2002;169(1):581–586.
- Liu J, Beller DI. Distinct pathways for NF-kappa B regulation are associated with aberrant macrophage IL-12 production in lupus- and diabetes-prone mouse strains. J Immunol. 2003;170(9):4489–4496.
- Kawada N, Moriyama T, Ando A, Koyama T, Hori M, Miwa T, Imai E. Role of intron 1 in smooth muscle alpha-actin transcriptional regulation in activated mesangial cells in vivo. Kidney Int. 1999;55(6):2338–2348.
- Bussolati B, Mariano F, Biancone L, Foa R, David S, Cambi V, Camussi G. Interleukin-12 is synthesized by mesangial cells and stimulates platelet-activating factor synthesis, cytoskeletal reorganization, and cell shape change. Am J Pathol. 1999;154(2):623–632.
- Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87(3):847–858.