Abstract
Cytomegalovirus has been implicated in the pathogenesis of transplant renal artery stenosis. However, the clinical course of this infection-associated transplant renal artery stenosis and its response to antiviral treatment is still unclear. We report a patient with transplant renal artery stenosis associated with an acute cytomegalovirus infection, which resolved following ganciclovir administration without the need for surgical or angiographic intervention. Serological testing revealed positive titers of anti- cytomegalovirus IgM and IgG antibodies. Renal allograft Doppler study findings were compatible with critical renal artery stenosis. Renal allograft angiography disclosed a critical circular stenosis. Following the intravenous ganciclovir administration, dramatically decreased Doppler ultrasound parameters along with the absence of parvus/tardus waveform pattern revealed the resolution of the stenosis. Moreover, the serological testing was negative for anti-cytomegalovirus IgM antibody, but anti-cytomegalovirus IgG antibody was positive. This report not only implies the causative possible relationship between acute cytomegalovirus infection and transplant renal artery stenosis, but it also highlights the importance of this complication when managing a renal transplant recipient with signs of allograft arterial stenosis.
INTRODUCTION
Transplant renal artery stenosis (TRAS) is an important cause of hypertension and renal allograft dysfunction occurring in 1–23% of the kidney transplant recipients.Citation[1] The pathogenesis of TRAS is related to the renal artery intimal injury and endothelial damage during allograft arterial anastomosis.Citation[2] An arterial inflammatory process with subsequent endothelial damage may also lead to diffuse renal artery stenoses late after transplant.Citation[1]
Cytomegalovirus (CMV) has been implicated in the pathogenesis of various vasculopathies. This infection has been reported as a cause of post-atherectomy coronary artery restenosis,Citation[3] accelerated vasculopathy of cardiac allograft,Citation[4] and coronary artery intimal thickening in animal models for cardiac transplant.Citation[5] Transient carotid stenosis with intimal-medial thickening has been reported during an episode of acute CMV infection.Citation[6] A retrospective study also found an association between CMV infection following renal transplantation and TRAS.Citation[7,Citation8] However, the clinical course of this infection-associated TRAS and its response to antiviral treatment is still unclear. We report a patient with TRAS associated with an acute CMV infection. To our knowledge, this report represents the first case of CMV-associated TRAS, which resolved with specific anti-CMV chemotherapy.
CASE REPORT
A 42-year-old female presented to the central university hospital with dyspnea and accelerated hypertension 13 months following a living-related renal transplant. The cause of her end stage renal disease was chronic pyelonephritis and nephrolithiasis. Post-surgical treatment included immunosuppressive therapy with mycophenolate mofetil, cyclosporine, and corticosteroid. Her blood pressure was within normal limits during her regular post-transplant visits. She had a history of fever, malaise, and anorexia for three weeks.
Upon admission, physical examination revealed a blood pressure of 160/115 mmHg and body temperature of 37.5°C. Laboratory findings were as follows: serum creatinine, 1.2 mg/dL; urea, 65 mg/dL; plasma sodium, 140 mEq/L; serum potassium, 4.1 mEq/L; calcium, 9.4 mg/dL; phosphorus, 5.7 mg/dL; uric acid, 8.2 mg/dL; white blood cell count, 4200/μL; hemoglobin, 12.6 mg/dL; platelets count, 201,000/μL; prothrombin time, 13 sec; and cyclosporine blood level, 149 ng/mL. Serological testing for parvovirus B19 was unrevealing; however, positive titers of anti-CMV IgM and IgG antibodies were detected. A review of pre-transplant records revealed that both the patient and donor had a positive anti-CMV IgG but negative anti-CMV IgM antibody before transplant. Renal allograft ultrasound and Doppler study disclosed a normal sized kidney (length: 130 mm) with a parvus-tardus waveform pattern in spectral study of the inter-lobar arteries. Resistive index (RI) and acceleration time (AT) of the inter-lobar arteries were 0.50 and 160 ms, respectively. Peak systolic velocity (PSV) of 131 cm/s in external iliac artery and 450 cm/s at renal artery anastomotic site was reported as well. Doppler study findings were compatible with critical renal artery stenosis.
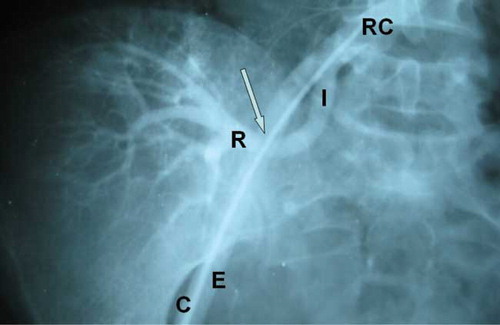
Anti-hypertensive therapy with an oral beta-blocker (atenolol) and calcium channel blocker (amlodipine) was begun. Renal allograft angiography disclosed a 5 mm critical circular stenosis (see ). With the clinical suspicion of acute CMV infection, treatment with intravenous ganciclovir (300 mg daily) was started and continued for 12 days. Within three months, her blood pressure diminished gradually, and she continued on amlodipine (5 mg/day).
Figure 1. The internal iliac artery is anastomosed end-to-end to the renal allograft artery. There is a significant circular stenosis, 5 mm in length at the site of anastomosis (arrow). This is overshadowed by the external iliac artery. Abbreviations: right common iliac artery (RC), internal iliac artery (I), renal artery (R), external iliac artery (E), catheter (C).
Doppler ultrasound performed five months following the anti-CMV therapy revealed the following parameters: RI, 0.60; PSV of external iliac artery, 110 cm/s; PSV at the anastomotic site of the renal artery, 200 cm/s; and AT in inter-lobar arteries, 0.75 ms. In addition, no parvus/tardus waveform pattern was detected during spectral study of the inter-lobar arteries. On one-year follow-up, her blood pressure was normal (120/70 mmHg) and serum creatinine was 1.1 mg/dL. The serological testing was negative for anti-CMV IgM antibody but anti-CMV IgG antibody was positive.
DISCUSSION
As TRAS is a major cause of renal allograft loss, its early detection and effective treatment may enhance graft and thus patient survival.Citation[1] Multiple factors are thought to contribute to the development of TRAS, such as surgical technique, type of allograft, immunologic factors, and CMV infection.Citation[2] TRAS usually presents with hypertension or graft dysfunction.Citation[1,Citation2] The severity of TRAS is assessed by measuring PSV at the stenotic and RI at the post-stenotic intrarenal arteries.Citation[1] Nevertheless, angiography provides a definite diagnosis.Citation[1] In our patient, allograft Doppler parameters along with angiographic findings were compatible with a critical TRAS. An acute CMV infection was confirmed with positive serology (IgM and IgG antibodies), and anti-CMV chemotherapy (ganciclovir) was subsequently administered. The stenosis disappeared following the ganciclovir administration, without need for surgical intervention, indicating a plausible association between acute CMV infection and TRAS.
Due to the ubiquitous nature of the virus, a large number of donors and recipients had latent CMV infection at the time of transplant.Citation[9] Studies have shown that CMV infection may result in reduced allograft survival.Citation[9] For instance, CMV inclusions have been identified in renal allograft specimens from posttransplant glomerulonephropathy.Citation[10]
As mentioned above, CMV has been implicated in the pathogenesis of various vasculopathies. It is postulated that CMV infection results in vascular damage by direct cytotoxicity or mitogenic action of viral gene products on the endothelial cells, vascular smooth muscle cells, and fibroblasts.Citation[7,Citation11] Infected endothelial cells induce vascular inflammation, thrombogenesis, and growth factor release, which in turn stimulate fibroblasts and smooth muscle cells (SMC) leading to accelerated narrowing of the vascular lumen.Citation[12] Furthermore, the inhibition of growth suppressor factor p53 is another possible mechanism by which CMV induces SMC proliferation.Citation[13] The migration of SMCs is also triggered by CMV chemokines, enhancing vascular myo-intimal thickening.Citation[14]
The present report demonstrated an acute TRAS episode coincidental with an acute CMV infection. Interestingly, the stenosis disappeared following anti-CMV chemotherapy without the need for surgical or angiographic intervention. Such an association may suggest a possible causative relationship between acute CMV infection and TRAS. We conclude that CMV infection may induce serious vascular damage such as transplant artery stenosis in kidney allograft recipients. This complication should be considered when managing a renal transplant recipient with signs of allograft arterial stenosis.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
REFERENCES
- Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15:134–141.
- Patel NH, Jindal RM, Wilkin T, Renal arterial stenosis in renal allografts: Retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001;219:663–667.
- Zhou YF, Leon MB, Waclawiw MA, Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996;335:624–630.
- Koskinen PK, Nieminen MS, Krogerus LA, Cytomegalovirus infection accelerates cardiac allograft Epstein vasculopathy: Correlation between angiographic and endomyocardial biopsy findings in heart transplant patients. Transplant Int. 1993;6:341–347.
- Lemström K, Koskinen P, Krogerus L, Daemen M, Bruggeman C, Häyry P. Cytomegalovirus antigen expression, endothelial cell proliferation, and intimal thickening in rat cardiac allografts after cytomegalovirus infection. Circulation. 1995;92:2594–2604.
- Hirabayashi Y, Ishii T, Kodera T, Fujii H, Munakata Y, Sasaki T. Acute cytomegalovirus infection and transient carotid intimal-medial thickening in a young, otherwise healthy woman. J Clin Microbiol. 2003;41:3978–3980.
- Pouria S, State OI, Wong W, Hendry BM. CMV infection is associated with transplant renal artery stenosis. QJM. 1998;91:185–189.
- Audard V, Matignon M, Hemery F, Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant. 2006;6:95–99.
- Weikert BC, Blumberg EA. Viral infection after renal transplantation: Surveillance and management. Clin J Am Soc Nephrol. 2008;3:S76–S86.
- Birk PE, Chavers BM. Does cytomegalovirus cause glomerular injury in renal allograft recipients?. J Am Soc Nephrol. 1997;8:1801–1808.
- Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–750.
- Alcami J, Barzu T, Michelson S. Induction of an endothelial cell growth factor by human cytomegalovirus infection of fibroblasts. J Gen Virol. 1991;72:2765–2770.
- Speir E, Modali R, Huang ES, Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394.
- Streblow DN, Soderberg-Naucler C, Vieira J, The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–520.