Abstract
Background. Vascular calcification is an important complication that worsens the prognosis for dialysis patients, although its detailed molecular mechanisms are still unknown. Methods. We produced a rat model for vascular calcification with hyperphosphatasemia and hyperparathyroidism, performing a 5/6 nephrectomy and providing a high-phosphorus, low-calcium diet for eight weeks. We examined mRNA obtained from the calcified aortae using microarray analysis, and searched for alterations in gene expression specifically in the calcified lesions. Results. Medial calcification was demonstrated in the abdominal aorta of 12 out of 42 hyperparathyroidism rats. In the aortae of hyperparathyroid rats with vascular calcification, the genes for heparan sulfate proteoglycans, including perlecan, were found to be down-regulated using microarray analysis and real time PCR. Immunohistochemistry also demonstrated reduced production of perlecan in the aortae of hyperparathyroid rats. Discussion. Perlecan is a major component of the vascular wall basement membrane and may play a role in protecting vascular smooth muscle cells from inflammatory cells and various toxins. It has also been reported that heparan sulfate chains may inhibit osteogenesis. Our findings indicate that perlecan may protect vascular smooth muscle cells from various factors that promote vascular calcification. Conclusions. It may be that reduced expression of perlecan in the calcified aortae of hyperparathyroid rats is a risk factor for vascular calcification.
INTRODUCTION
It is well known that patients with chronic kidney disease (CKD), especially end-stage renal disease (ESRD), are susceptible to severe vascular calcification (VC) in systemic blood vessels.Citation[1] Calcified arteries lose elasticity, exacerbating ischemia of the perfused organ, and increase cardiac afterload. VC-associated cardiovascular diseases accordingly account for the cause of death in about half of dialysis patients.Citation[2–4] Known risk factors for VC include aging, diabetes mellitus, and hypertension. Recently, secondary hyperparathyroidism (HPT) and hyperphosphatasemia have become hot topics as risk factors for VC typical of ESRD patients.Citation[5,Citation6]
On the other hand, the molecular mechanisms of VC have yet to be elucidated. Pathological analyses have repeatedly demonstrated that bone-associated proteins are expressed in calcified arteries,Citation[7–9] suggesting that VC is in some ways similar to developmental bone mineralization,Citation[10,Citation11] though the evidence is not conclusive.
To investigate the mechanism of VC, we produced a rat model for VC with hyperphosphatasemia and HPT, performing a 5/6 nephrectomy and providing a high- phosphorus (P), low-calcium (Ca) diet for eight weeks. We compared mRNA obtained from the calcified aortae with mRNA from non-calcified aortae using microarray analysis and searched for alterations in gene expression specifically in the calcified lesions. In aortae with VC, we found reduced expression of perlecan, the major heparan sulfate proteoglycan (HSPG) present in arteries, and a major component of the basement membrane and extracellular matrix in cartilage.
MATERIALS AND METHODS
Rat Model for Vascular Calcification with Hyperphosphatasemia and Hyperparathyroidism
To create uremia in eight-week-old male Sprague-Dawley rats, we performed 5/6 nephrectomies under intraperitoneal pentobarbital anaesthesia (50 mg/kg body weight). Rats were fed a normal diet (0.9% P, 1.12% Ca) until one week after the procedures and then switched to a high P, low Ca diet (1.2% P, 0.4% Ca) for eight weeks to induce HPT (HPT group, n = 42). Control group rats (Nx group, n = 16) were fed a normal diet for nine weeks after surgery. Feed was obtained from Oriental Yeast, Inc. (Chiba, Japan). Animal care and experimental use were approved by the Animal Studies Committee of Wakayama Medical University, Wakayama, Japan.
Blood Tests
Blood samples were taken from the jugular vein at the time of sacrifice. Serum samples were assayed for blood urea nitrogen (BUN), creatinine (Cr), albumin, calcium (Ca), phosphorus (P), and intact parathyroid hormone (iPTH). Serum iPTH levels were measured using a two antibody method with a rat iPTH enzyme-linked immunosorbent assay kit (Immutopics, Inc., California, USA). Other assays were performed using an automated analyser (DRI-CHEM3500V, Fuji Film, Tokyo, Japan).
Tissue Preparation and Identification of Vascular Calcification
Nine weeks after 5/6 nephrectomy, we sacrificed animals under anaesthesia and resected the aortae from the ascending aorta to the iliac bifurcation. We then removed extraneous tissue by blunt dissection and divided the aorta into three pieces: the thoracic aorta for RNA extraction and the aortic arch and abdominal aorta for histological analysis.
For histological analysis, the aortic arch and abdominal aorta were fixed with 4% paraformaldehyde PBS for 8 h and processed for paraffin embedding. To identify VC, we performed hematoxylin and eosin staining and von Kossa staining (silver nitrate plus nuclear fast red) according to the standard protocols. We used RNA from the thoracic aorta from HPT rats with confirmed VC (HPT with VC; n = 12) for microarray analysis and quantitative real-time PCR, as described below.
RNA Isolation
Total RNA was isolated from the aortas of HPT rats with medial calcification and Nx rats using TRIzol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer's instructions. RNA cleanup was performed using the RNeasy MinElute cleanup kit (Qiagen, Valencia, California, USA). RNA concentrations were quantified by absorbance at 260 nm. RNA integrity was assessed by electrophoresis on a 1% agarose gel.
Microarray Analysis
RNA extracted from the thoracic aortae of HPT rats with VC (HPT with VC; n = 12) and Nx rats without calcification (Nx; n = 16) was used for microarray analysis. We randomly selected five samples from each group.
We placed an order with Filgen Inc. (Nagoya, Japan) for the microarray analyses. Briefly, fluorescence-labeled antisense RNA was synthesized by direct incorporation of Cy3-UTP or Cy5-UTP (GE Healthcare Bio-Sciences, Japan) using 2 μg of each RNA sample and an RNA Transcript SureLABEL Core Kit (TaKaRa BIO Inc., Tokyo, Japan). Labeled antisense RNAs were hybridized simultaneously to the microarray chip (FilgenArray Rat 27k, Filgen Inc., Nagoya, Japan). Array hybridization was performed according to the manufacturer's instructions. Fluorescence images of the hybridized microarrays were obtained using a GenePix 4000B scanner (Axon Instruments Ltd., Inverurie, Scotland). An Array-Pro Analyzer Ver4.5 (Media Cybernetics, Inc., Silver Spring, Maryland, USA) was used to determine the signal intensity of each spot and its local background. The net intensity was calculated by subtracting the mean intensity of all pixels within the local background area from the mean intensity of all pixels within the spot areas. Biases in net intensity were normalized by locally weighted linear regression analysis. Analyzed data were selected using a MicroArray Data Analysis Tool (Filgen Inc.). Normalized intensity ratios more than 2.0 or less than 0.5 were considered significant.
Quantitative Real-Time PCR for Proteoglycan-Related Genes
Single-strand cDNA was generated by reverse transcription with an Omniscript kit (Qiagen, Valencia, California, USA) from 1 μg of total RNA using random hexamer primers. mRNA for osteoblast marker genes and proteoglycan-related genes was detected by real-time PCR using an ABI Prism 7500 sequence detection system and the Power SYBR® Green PCR Master Mix (PE Biosystems, Foster City, California, USA). Data were normalized to rat glyceraldehyde-3-phophate dehydrogenase (GAPDH) as an endogenous control. The forward and reverse primer sequences were as follows:
perlecan, 5′-CCCTGGCAACAGCTTCTCTA-3′ and 5′-ATGGCCATCCTGTAGTCCAA-3′;
collagen, 18 5′-GCCCGCATCTTTTCTTTCG-3′ and 5′-CGTCTCACAGTAGCTCTCCATCA-3′;
syndecan, 4 5′-TCTTCGCCGTTTTCCTGATC-3′ and 5′-CGTAACTGCCTTCATCCTTCTTC-3′;
CSPG2, 5′-GAATGACGTCCCCTGCAACTA-3′ and 5′-TGGCCGCAAGCAACTGT-3′;
decorin, 5′-AGCAACCCTGTCCGGTATTG-3′ and 5′-TGGTAGAGCGCCCGAAGAC-3′;
biglycan, 5′-CTTCCGCTGCGTCACTGA-3′ and 5′-GGTGGCTACCACTGCTTCTACTTC-3′;
lumican, 5′-GGCTGTCTCGGCTTCTCTGA-3′ and 5′-TTGCTCATCTGATTGAAGCTCAAG-3′;
mimecan, 5′-CGATGATACATTCTGCAAGGCTAA-3′ and 5′-CCAGGCGGATCTCTTCCAT-3′; and
GAPDH, 5′-GTGGACCTCATGGCCTACAT-3′ and 5′-GGATGGAATTGTGAGGGAGA-3′.
The thermal profile for PCR was 50°C for 2 min, 95°C for 10 s, and 60°C for 1 min. Dissociation curves were checked using ABI prism 7500 SDS software to ensure that the signal produced was specific to the target sequence. Run data were exported to MS Excel for calculation of unknown copy numbers from the standards. Gene expression data are expressed as percent of control.
Immunohistochemistry
Immunohistochemical staining was performed on 4% paraformaldehyde-fixed, paraffin-embedded specimens from the aortic arch and abdominal aorta using anti-rat perlecan antibodies (1:100 dilution). Immunohistochemical staining was performed with the Ventana HX system (Ventana, Yokohama, Japan), using protease I to retrieve epitope. Goat anti-rat perlecan (L20) polyclonal antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA; catalogue number sc-27449).
Primary Culture of Rat Aortic Smooth Muscle Cells
Rat aortic smooth muscle cells (RASMCs) were separated from the aortae and extracted using an explant method originally described by Campbell and Campbell.Citation[12] Briefly, the medial layer of the thoracic aorta was excised, dissected into 1 mm3 pieces, and placed in 100 mm dishes. The pieces were cultured for several weeks in Dulbecco Modified Eagle Medium (DMEM) containing 4.5 g/L glucose, 10 mmol/L sodium pyruvate, and 20% foetal bovine serum supplemented with 25 μg/mL gentamicin and 50 ng/mL amphotericin B, at 37°C in a humidified atmosphere containing 5% CO2. Cells that migrated from the explants were collected and maintained in DMEM containing 10 mmol/L sodium pyruvate supplemented with 10% foetal bovine serum (culture medium). Cells up to passage 5 were used for the experiments. Media were replaced every two days. To ensure that explanted cultures were purely SMCs, cells were stained for SMC α-actin using the 1A4 smooth muscle actin antibody (NeoMarkers, Fremont, California, USA) at a 1:200 dilution.
In Vitro Calcification
Induction of calcification of the RASMCs was performed by the procedure described by Jono et al.Citation[11] with minor modifications. In the calcifying group, P was added to the culture medium in the form of NaH2PO4 and Na2HPO4 in a 1:2 ratio to achieve a final P concentration of 5 mM. In the control group, the final P concentration was 0.9 mM because the DMEM contained phosphorus originally. After 14 days of incubation, total RNA was isolated from the cells using TRIzol reagent, and real-time PCR was performed. We used von Kossa staining to confirm induction of calcification as mineral-containing deposits in the cell layer.
Statistical Analyses
Data are presented as mean ± SD. Statistically significant differences were determined by Student's t-test. p values less than 0.05 were considered significant.
RESULTS
Rat Model for Vascular Calcification with Hyperphosphatasemia and Hyperparathyroidism
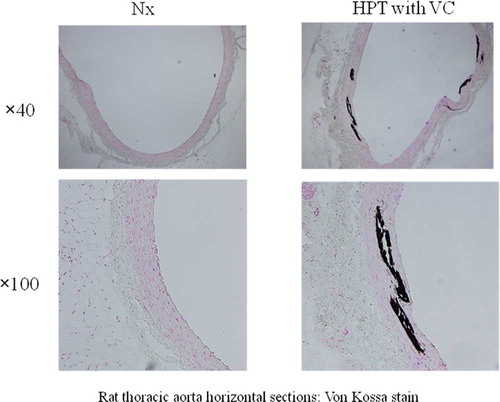
HPT rats weighed significantly less than the Nx rats (control group). Blood tests showed significantly lower Ca levels, higher P levels, and higher iPTH levels in HPT rats than in Nx rats. No difference was seen in the degree of the renal damage between groups (see ). Medial calcification was demonstrated in the abdominal aorta in 12 out of 42 HPT rats (HPT with VC, n = 12) (see ). No VC was seen in Nx rats (Nx, n = 16). No differences were seen in blood test results between HPT with VC (n = 12) and HPT without VC (n = 30) (data not shown).
Table 1 Body weight and serum biochemistry
Figure 1. VC occurs mainly in the medial layer of the aorta. Medial calcification was demonstrated in the abdominal aorta in 12 out of 42 HPT rats (HPT with VC: n = 12). No VC was found in the Nx rats (control group) (Nx; n = 16). Abbreviations: Nx = nephrectomized, HPT = hyperparathyroidism, VC = vascular calcification.
Comparison of Gene Expression Profiles in Vascular Calcification Using Microarray Analysis
The results of microarray analysis showed that 42 genes were up-regulated and 68 genes down-regulated in the aortae of HPT with VC animals when compared with Nx. Extracts are shown in and . Of the down-regulated genes, we focused on perlecan, one of the heparan sulfate proteoglycans and a major component of vascular wall basement membrane, because earlier studies reported a decrease of perlecan in atherosclerotic lesionsCitation[13] and in diabetic arteries.Citation[14] It has also been reported that heparan sulfate/heparin inhibits bone mineralization and VC in vitro.Citation[15,Citation16]
Table 2 DNA microarray data (2 up)
Table 3 DNA microarray data (2 down)
First, to investigate whether perlecan is the only proteoglycan to have reduced expression in VC, we examined changes in the expression of other proteoglycan genes using microarray analysis (see ). As reported in previous studies of atherosclerotic lesionsCitation[13] and diabetic arteries,Citation[14] mRNA of HSPGs tended to decrease. On the other hand, mRNA of chondroitin/dermatan sulfate proteoglycans (CS/DSPGs) and keratan sulfate proteoglycans (KSPGs) was either unchanged or tended to increase.
Table 4 DNA microarray data (genes related to proteoglycans)
Confirmation Using Real-Time PCR
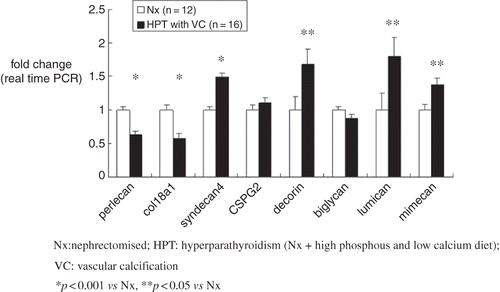
Using both real-time PCR as well as microarray analysis, we confirmed reduced expression of perlecan in the aortae of HPT with VC animals (see ). Furthermore, expression of collagen 18, an HSPG and vascular wall basement membrane component, was also decreased. Expression of syndecan 4, an HSPG but not a basement membrane component, tended to increase. For the CSPG/DSPGs, no significant changes were seen in CSPG2 and biglycan, whereas expression of decorin increased significantly. Expression of lumican and mimecan, both KSPGs, also increased significantly. In particular, expression of perlecan was reduced in all aortae with VC. In the aortae of HPT without VC animals, perlecan tended to be reduced to a similar extent, although the difference between HPT without VC and Nx was not significant (data not shown).
Figure 2. RNA extracted from the thoracic aortae of HPT rats with vascular calcification (HPT with VC; n = 12) and Nx rats without calcification (Nx; n = 16) was used for real-time PCR. Reduced expression of perlecan was seen in HPT rats with VC, confirming the microarray analysis findings. Furthermore, expression of collagen 18, one of the HSPGs and a vascular wall basement membrane component, was also decreased. Expression of syndecan 4, an HSPG but not a basement membrane component, tended to increase. For the CSPG/DSPGs, no significant changes were seen in CSPG2 and biglycan, whereas expression of decorin increased significantly. Expression of lumican and mimecan, both KSPGs, also increased significantly. Data were normalized to rat glyceraldehyde-3-phophate dehydrogenase (GAPDH) as an endogenous control. Gene expression data are presented as percent of control. Abbreviations: Nx = nephrectomized, HPT = hyperparathyroidism (Nx + high-phosphorus, low-calcium diet), VC = vascular calcification. *p < 0.001 vs. Nx, **p < 0.05 vs. Nx.
Immunohistochemistry of Perlecan in the Aorta
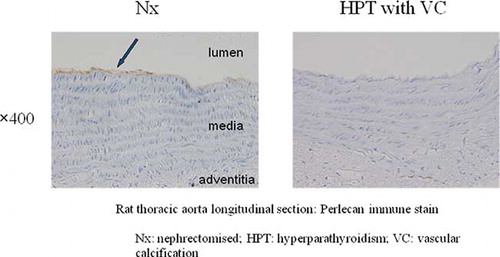
Immunohistochemistry using anti-perlecan antibodies showed positive staining in the basement membrane under endothelial cells in the Nx aorta, whereas no positive staining was detected in HPT. These results indicate that not only perlecan-related mRNA but also its products are decreased in aortae with VC (see ).
Figure 3. Immunohistochemical examination using anti-perlecan antibodies showed positive staining in the basement membrane (arrow) under endothelial cells in the Nx aorta, whereas no positive staining was seen in the HPT aorta. Abbreviations: Nx = nephrectomized, HPT = hyperparathyroidism, VC = vascular calcification.
Reduced Expression of Perlecan in the In Vitro Calcification Model
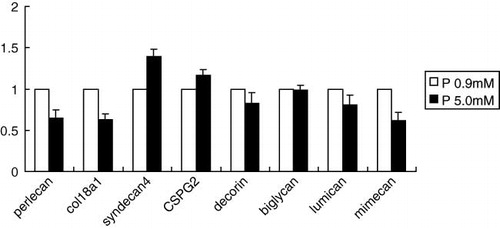
RASMCs cultured at P 5 mM showed extensive mineral precipitations, demonstrated using von Kossa staining (see ). This calcification was inhibited by 1 mM phosphonoformic acid (PFA), as previously reported by Jono et al.Citation[11] Real-time PCR demonstrated reduced expression of HSPGs (perlecan and collagen 18) in the calcifying group, although there were no significant changes in decorin and lumican expression (see ).
Figure 4. In the calcifying group, P was added to the culture medium in the form of NaH2PO4 and Na2HPO4 in a 1:2 ratio to achieve a final P concentration of 5 mM. In the control group, the final P concentration was 0.9 mM because the DMEM contained P originally. After 14 days of incubation, RASMCs cultured at P 5 mM showed extensive von Kossa staining and mineral precipitation. Abbreviations: RASMCs = rat aortic smooth muscle cells, P = phosphorus.
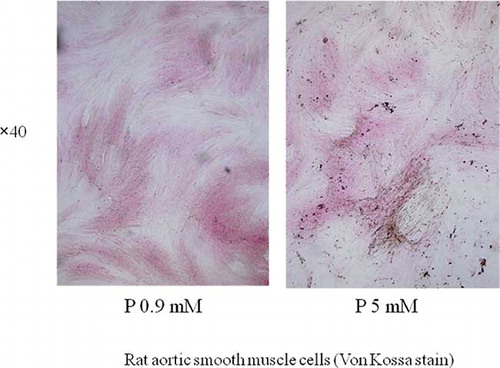
Figure 5. After 14 days of incubation, total RNA was isolated from the cells and real-time PCR was performed. Real-time PCR showed reduced expression of the HSPGs perlecan and collagen 18 in the calcifying group, but no significant changes were seen in decorin and lumican. Data were normalized to rat glyceraldehyde-3-phophate dehydrogenase (GAPDH) as an endogenous control. Data are presented are mean-fold increase in gene expression from five experiments ± SD. Abbreviation: P = phosphorus.
Discussion
In this report, we demonstrated using microarray analysis and real-time PCR that mRNA expression of perlecan, the major HSPG in arteries, is reduced in aortae with VC induced by hyperphosphatasemia and HPT. Perlecan is a major HSPG, which is widely found in basement membranes and cartilage.Citation[17] Three very long heparan sulfate chains (molecular weight: together about 360 kDa) attach to its large core protein (molecular weight: about 470 kDa)Citation[18]; therefore, perlecan is charged negatively in abundance by sulfate groups. In the glomerular basement membrane, perlecan is thought to repel negatively charged proteins, including albumin, thereby preventing their filtration. As a result, it plays a role in glomerular permselectivity.Citation[19] In addition, as a property specific to HSPGs, perlecan HS chains bind and modify the effects of heparin-binding growth factors and directly or indirectly influence cellular interactions with the extracellular matrix (ECM), which is important in the control of cell behavior and phenotype.Citation[20,Citation21]
In arteries, perlecan is a major HSPG, found in subendothelial basement membrane and medial ECM as demonstrated by immunostaining.Citation[21–23] The role of perlecan within arteries has not been fully elucidated, although several possibilities have been reported:
it works to maintain the arterial structure as part of the extracellular matrix;
it helps to stabilize the endothelial barrier as a basement membrane component along with endothelial cellsCitation[17,Citation24];
it modifies the effects of growth factors such as FGF and PDGF, and is a potent modulator of cellular phenotype and proliferation (inhibiting the proliferation of smooth muscle cells and promoting endothelial cells)Citation[21,Citation23,Citation24]; and
it has local anticoagulant activity because of its structural similarity to heparin.Citation[21]
Based on the above effects, an anti-atherogenic role has been proposed for perlecan.Citation[13,Citation21,Citation23,Citation24] It is interesting that reduced expression of perlecan was seen in calcified arteries. Decreased production of HSPGsCitation[13,Citation25,Citation26] and increased production of CSPGs and DSPGs,Citation[14,Citation27–29] has been reported in arteries with atherosclerotic plaque and arteriosclerotic lesions. Equally of interest is the finding of decreased production of HSPGs in arteries from patients with diabetes,Citation[14,Citation29] a strong risk factor of VC equal to renal failure.
As a homozygous deletion of perlecan in mice is lethal due to bone and neurological malformations,Citation[30] perlecan is known to be essential for normal bone formation and neurological development. Heparin, which has components similar to heparan sulfate, inhibits the maturation of osteoblastsCitation[31] and thus induces osteoporosis.Citation[32] Jono et al. suggested that phenotypic transition of vascular smooth muscle cells to osteoblast-like cells may be involved in the mechanism of VC.Citation[11] The heparan sulfate chains of perlecan may protect smooth muscle cells from phenotypic transition to osteoblast-like cells in VC lesions. Furthermore, Yang et al. demonstrated using an in vitro VC model with high P concentrations that heparin inhibits osteoblast differentiation and mineralization in primary cultures of bovine aortic smooth muscle cells (BASMC). In their study, these effects were found not to be dependent upon heparin's anticoagulant activity, but rather sulfation was found to be a major determinant of heparin's ability to inhibit BASMC mineralization.Citation[15]
BMP2 protein has been found in VC lesions, indicating that a BMP2 signaling pathway is implicated in VC formation. Jiao et al. demonstrated that HSPGs directly regulates BMP2-mediated transdifferentiation of C2C12 myoblasts into osteoblasts. They also showed that HSPGs mediates BMP2 internalization and modulates BMP2 osteogenic activity.Citation[16]
Perlecan is a major component of the vascular wall basement membrane, which protects smooth muscle cells, is negatively charged, and repels various molecules. It follows that perlecan protects smooth muscle cells from various toxic substances and circulating inflammatory cells. One study found that perlecan inhibits monocyte binding to vascular endothelium.Citation[33] These data suggest that perlecan and heparan sulfate may be inhibitory factors for VC.
In this study, we found that expression of perlecan is reduced in calcified arteries, but no changes were seen in the expression of other proteoglycans, indicating that changes are specific to HSPGs. We also found that the expression of markers specific to smooth muscle cells such as actin was also not decreased (data not shown). What then is the trigger for decreased perlecan in calcified arteries? In the in vitro calcification model of RASMCs, it was shown that the expression of perlecan and collagen 18 of RASMCs was decreased after the addition of P. Moreover, in the aortae of HPT rats with or without VC, the expression of perlecan tended to decrease, although not significantly. These results suggest that reduced perlecan expression begins before the formation of calcified lesions. Further studies are required to determine whether this is due to direct reduction of perlecan induced by P or is the result of loss of the smooth muscle cell phenotype. P-induced phenotypic transition to osteoblast-like cells might cause a decreased expression of perlecan by RASMCs and accelerate VC.
Both inhibitory factors and accelerator factors such as P likely play a part in the mechanism of VC.Citation[34] RASMCs readily transform into osteoblast-like cells and mineralize in vitro after only 14 days following the addition of P, as shown in the in vitro experiments. In our in vivo study, however, not all HPT rats developed VC of the aorta, despite similar severities of hyperphosphatasemia and HPT. It may be that the arterial endothelium and basement membrane structure protect smooth muscle cells from hyperphosphatasemia and inhibit the development of VC.
In this study, we confirmed reduced expression of perlecan in VC models both in vivo and in vitro. Further studies are required to determine whether reduced expression of perlecan is directly associated with the promotion of VC. However, if the heparan sulfate chains in perlecan are protective against VC, reduction of perlecan expression in aorta of HPT rats may exacerbate VC. In summary, HSPGs, particularly perlecan, were down-regulated in the calcified aortae of a uremic rat model with hyperphosphatasemia and HPT. Reduced expression of perlecan was also confirmed in an in vitro P-induced calcification model of RASMCs. These results suggest that heparan sulfate protects vascular smooth muscle cells from various VC promotion factors. Reduced expression of perlecan in the calcified aorta of HPT rats may therefore be a risk factor for VC.
ACKNOWLEDGMENTS
The author has never had involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
REFERENCES
- Goodman WG, Goldin J, Kuizon BD, Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483.
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001;38:938–942.
- London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740.
- Raggi P, Boulay A, Chasan-Taber S, Cardiac calcification in adult hemodialysis patients: A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701.
- Goodman WG, London G, Amann K, Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579.
- Chertow GM, Raggi P, Chasan-Taber S, Bommer J, Holzer H, Burke SK. Determinants of progressive vascular calcification in hemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–1496.
- Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809.
- Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402.
- Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176.
- Steitz SA, Speer MY, Curinga G, Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154.
- Jono S, McKee MD, Murry CE, Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17.
- Campbell JH, Campbell GR. Culture techniques and their applications to studies of vascular smooth muscle. Clin Sci (Lond). 1993;85:501–513.
- Tran PK, Agardh HE, Tran-Lundmark K, Reduced perlecan expression and accumulation in human carotid atherosclerotic lesions. Atherosclerosis. 2007;190:264–270.
- Edwards IJ, Wagner JD, Vogl-Willis CA, Litwak KN, Cefalu WT. Arterial heparan sulfate is negatively associated with hyperglycemia and atherosclerosis in diabetic monkeys. Cardiovasc Diabetol. 2004;29:3–6.
- Yang L, Butcher M, Simon RR, Osip SL, Shaughnessy SG. The effect of heparin on osteoblast differentiation and activity in primary cultures of bovine aortic smooth muscle cells. Atherosclerosis. 2005;179:79–86.
- Jiao X, Billings PC, O'Connell MP, Kaplan FS, Shore EM, Glaser DL. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. J Biol Chem. 2007;282:1080–1086.
- Iozzo RV, Cohen IR, Grässel S, Murdoch AD. The biology of perlecan: The multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302:625–639.
- Kimura S, Cheng J, Toyoshima K, Oda K, Saku T. Basement membrane heparan sulfate proteoglycan (perlecan) synthesized by ACC3, adenoid cystic carcinoma cells of human salivary gland origin. J Biochem. 1999;125:406–413.
- Groffen AJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant. 1999;14:2119–2129.
- Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99:2062–2070.
- Segev A, Nili N, Strauss BH. The role of perlecan in arterial injury and angiogenesis. Cardiovasc Res. 2004;63:603–610.
- Pillarisetti S. Lipoprotein modulation of subendothelial heparan sulfate proteoglycans (perlecan) and atherogenicity. Trends Cardiovasc Med. 2000;10:60–65.
- Kinsella MG, Tran PK, Weiser-Evans MC, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury: Role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler Thromb Vasc Biol. 2003;23:608–614.
- Paka L, Goldberg IJ, Obunike JC, Perlecan mediates the antiproliferative effect of apolipoprotein E on smooth muscle cells. An underlying mechanism for the modulation of smooth muscle cell growth? J Biol Chem. 1999;274:36403–36408.
- Stevens RL, Colombo M, Gonzales JJ, Hollander W, Schmid K. The glycosaminoglycans of the human artery and their changes in atherosclerosis. J Clin Invest. 1976;58:470–481.
- Pillarisetti S, Paka L, Obunike JC, Berglund L, Goldberg IJ. Subendothelial retention of lipoprotein (a). Evidence that reduced heparan sulfate promotes lipoprotein binding to subendothelial matrix. J Clin Invest. 1997;100:867–874.
- Tammi M, Seppala PO, Lehtonen A, Mottonen M. Connective tissue components in normal and atherosclerotic human coronary arteries. Atherosclerosis. 1978;29:191–194.
- Hollmann J, Schmidt A, von Bassewitz DB, Buddecke E. Relationship of sulfated glycosaminoglycans and cholesterol content in normal and arteriosclerotic human aorta. Arteriosclerosis. 1989;9:154–158.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: Changes in atherosclerosis and diabetes mellitus. Diabetologia. 1993;36:316–322.
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358.
- Bhandari M, Hirsh J, Weitz JI, Young E, Venner TJ, Shaughnessy SG. The effects of standard and low molecular weight heparin on bone nodule formation in vitro. Thromb Hemost. 1998;80:413–417.
- Sonawane S, Kasbekar N, Berns JS. The safety of heparins in end-stage renal disease. Semin Dial. 2006;19:305–310.
- Vogl-Willis CA, Edwards IJ. High glucose-induced alterations in subendothelial matrix perlecan leads to increased monocyte binding. Arterioscler Thromb Vasc Biol. 2004;24:858–863.
- Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567.