Abstract
Background. Sp1 is a ubiquitous transcription factor that mediates the fibrogenic factor transforming growth factor beta (TGF-β) signals through cooperation with Smad proteins. The transcriptional coactivator p300 is also suggested to play a role in Smad signal transduction. Methods. We investigated the immunohistochemical expression of Sp1 as well as the expression of pSmad2/3 and the coactivator p300 in 157 renal biopsy specimens from patients with various types of glomerulonephritis (GN). Correlations between immunohistochemical, clinical, and histologic parameters were performed. Results. Sp1 exhibited an increased glomerular and proximal tubular expression in all forms of GN compared to controls. The proximal tubular expression of Sp1 was significantly increased in proliferative GNs (p = 0.025), whereas in secondary GNs, there was a significant increase in the molecule's glomerular expression (p = 0.008). Sp1 correlated positively with pSmad2/3 and p300 expression in proximal tubules (r = 0.241, p = 0.018 and r = 0.244, p = 0.014, respectively), while in proliferative GNs, its expression correlated positively with pSmad2/3 expression in glomeruli (r = 0.32, p = 0.028). Sp1 glomerular and proximal tubular immunostaining correlated positively with serum creatinine levels (r = 0.265, p = 0.02 and r = 0.306, p = 0.006, respectively), while its proximal tubular expression showed a similar correlation with interstitial fibrosis (r = 0.213, p = 0.025). Sp1 was constantly detected in hyperplastic lesions and cellular crescents (each 100%), and very often in micro adhesions (94%) and segmentally or globally sclerotic areas (each 83%). Conclusions. This study documents the upregulation of Sp1 expression in glomeruli and proximal tubules of GN specimens. Our findings suggest a possible cooperation of Sp1 with pSmad2/3 and p300 in mediating renal injury as well as a possible role for this molecule in the pathogenesis and the progression of human GN.
INTRODUCTION
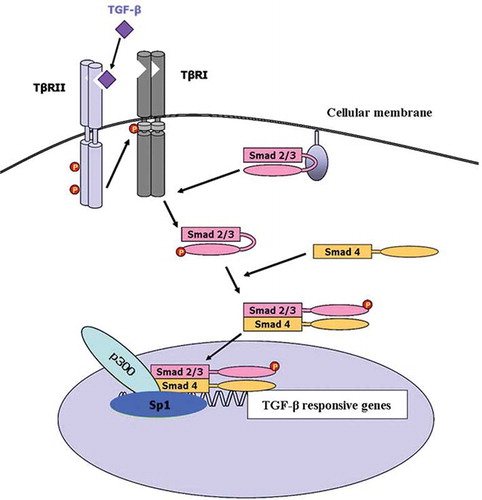
TGF-β is a multifunctional signaling protein that regulates apoptosis, cell cycle, differentiation, and extracellular matrix accumulation.Citation[1] It has been proposed to play a significant role in the progression of renal fibrosis in both human and experimental kidney diseases.Citation[2–4] The critical steps in the intracellular TGF-β signaling pathways are mediated by Smad proteinsCitation[5] (see ). Briefly, TGF-β initiates its cellular response by binding to the constitutively active TGF-β type II receptor (TβRII). Upon ligand binding, TβRII associates with and phosphorylates the type I receptor (TβRI).Citation[6] TβRI kinase then phosphorylates the receptor-regulated Smads (R-Smads; Smad2 and Smad3).Citation[7,Citation8] The phosphorylated Smad2/3 form heteromeric complexes with the common Smad (Co-Smad; Smad4) and translocate into the nucleus.Citation[7,Citation9–13] Smad complexes interact with DNA weakly and insufficiently to convey promoter selectivity.Citation[14] Thus, Smad complexes require other transcription factors and co-activators to target them to specific sequences. p300 is a transcriptional co-activator that appears to be involved in the transcriptional activation of Smad complexes.Citation[13–15] It possesses histone acetyltransferase activity, suggesting that transcriptional activation by Smad complexes may require altering nucleosome structure and remodeling of the chromatin.Citation[16] We have recently shown that the pSmad2/3-p300 pathway might play a pivotal role in the pathogenesis and progression of human glomerulonephritis (GN).Citation[17] Moreover, p300 might be involved in TGF-beta1/Smad-pathway-mediated type I collagen gene transcription in mouse mesangial cells.Citation[18]
Sp1 is a sequence-specific DNA-binding protein that is important for the transcription of many cellular and viral genes that contain GC boxes in their promoter regions.Citation[19] Many of the actions of TGF-β are mediated by Sp1 in cooperation with Smad proteinsCitation[20–25] (see ). Recent work demonstrated that Smad proteins interact with Sp1 and p300 to mediate TGF-β signals.Citation[26,Citation27] However, no data exist on the expression pattern of Sp1 in normal or diseased human kidneys.
In an effort to investigate the role of Sp1 in human GN and its possible interactions with pSmad2/3 and p300 in mediating renal injury, we examined the expression of these molecules using immunohistochemical methods in serial sections of a wide range of glomerulonephritides. We localized Sp1 expression to various specific pathologic lesions. Correlations were performed between the expression profile of these molecules and clinical parameters of renal function and histologic injury.
SUBJECTS AND METHODS
Patients
Percutaneous renal biopsies were obtained from 157 patients with various types of GN. Renal biopsies were performed for diagnostic purposes, and informed consent for the use of renal biopsy tissue, in excess of that required for diagnostic purposes, was obtained from the patients. Ninety-one patients had primary GN, and 66 patients secondary GN. In addition to taking into account the primary or the secondary nature of the glomerular disorder, GNs were further divided into proliferative (n = 86) and nonproliferative (n = 71). The non-proliferative group of primary GNs included minimal change glomerular disease (MCD, n = 10), membranous GN (MGN, n = 23), and focal segmental glomerulosclerosis (FSGS, n = 16), whereas the respective group in SLE GNs consisted of cases with mesangial changes (WHO class II, n = 10) and diffuse membranous GNs (WHO class V, n = 8). The proliferative group of primary GNs included acute diffuse proliferative GN [in detail, acute post-infectious GN (PI, n = 6), membranoproliferative GN (MPGN, n = 7)] and IgA nephropathy (n = 24), while the proliferative group of secondary GNs comprised those pauci-immune GN due to vasculitis (Pauci-Im GN, n = 23) as well as focal segmental SLE GN (WHO class III, n = 9) and diffuse proliferative SLE GN (WHO class IV, n = 16). The classification and clinical parameters of patients with GN are given in . As a control group, totally normal kidney sections from 15 kidneys resected for renal cell carcinoma were examined.
Table 1 Classification and clinical parameters of patients with glomerulonephritis
Immunohistochemistry
Immunohistochemical staining was performed on 4 μm-thick formalin-fixed paraffin embedded sections, using a biotin free, two-step, HRP-labeled detection system (DACO EnVisionTM). Sections were deparaffinized in xylene and rehydrated through graded alcohols. After quenching of endogenous peroxidase activity using a methanol/hydrogen peroxide solution (0.3% in TBS for 30 min), we proceeded to microwave heat-mediated antigen retrieval in 10 mM, pH = 6 citrate buffer and blockage of nonspecific binding by incubation in 10% normal horse serum in TBS (Vector Lab, Burlingame, California, USA) for 30 min at room temperature. Subsequently, sections were incubated for 40 minutes at 4oC with the primary antibodies. The primary antibodies used in our study were:
anti-Sp1 rabbit polyclonal antibody (sc-59) (Santa Cruz Biotechnology Inc., UK) at a dilution 1:100;
anti-pSmad2/3 rabbit polyclonal antibody (#3101) (Cell Signaling Technology Inc., USA) at a dilution 1:500; and
anti-p300 rabbit polyclonal antibody (sc-585) (Santa Cruz Biotechnology Inc., UK) at a dilution 1:500.
Sections were then incubated with a biotin free, two-step, HRP-labelled detection system (DACO EnVisionTM) for 30 min at room temperature, followed by the addition of 3,3' -diaminobenzidinetetrachlorohydrate (DAB), in order to achieve visualization of the antigens in question. The biotin-avidin/streptavidin system is often used as an amplification step to increase sensitivity. However, in some tissues, such as kidney, “nonspecific interactions might present a problem due to high levels of endogenous biotin-containing proteins.Citation[28] Thus, a biotin free detection system was utilized in our experiments. Finally, sections were rinsed, counterstained with hematoxylin, and mounted. In cases where the identification of proximal versus distal tubules was difficult, consecutive serial sections were stained so that any remnants of brush border became detectable. Previously Sp1-, pSmad2/3-, or p300-positive breast cancer tissue sections served as positive controls in each staining procedure, while negative controls included substitution of primary antibody with non-immune mouse IgG diluted at the same concentration for each antibody, or the omission of primary antibodies.
Quantification of Sp1, pSmad2/3, and p300 Immunostaining in Human Renal Biopsies
Serial sections were immunostained for each molecule. Nonglobally sclerosed glomeruli (mean, 15 ± 4.3; range, 10–25) were counted. The intensity of glomerular staining of Sp1, pSmad2/3, and p300 was evaluated according to the following 0 to 3 scale: 0, <10% cells with nuclear staining; 1, weak nuclear staining intensity or 11–50% of cells with nuclear staining; 2, moderate nuclear staining intensity and >50% of cells with nuclear staining, and 3, strong nuclear staining intensity and >50% of cells with nuclear staining. The staining status of glomerular lesions was also assessed, with immunopositivity being recorded when some degree of immunostaining was noticeable. When no staining was observed, the respective lesions were characterized as negative. The staining intensity of the different parts of the tubules was evaluated according to the following: 0, <10% cells with nuclear staining; 1, weak nuclear staining intensity or 11–50% of cells with nuclear staining; 2, moderate nuclear staining intensity and >50% of cells with nuclear staining, and 3, strong nuclear staining intensity and >50% of cells with nuclear staining. Sections were scored independently by two investigators (T.K. and A.N.) who were blinded to the patients' clinical profile.
Quantification of Histologic Injury in Human Renal Biopsies
The human renal biopsy specimens were reviewed and analyzed by an independent anatomic pathologist who was blinded to the quantification of Sp1, pSmad2/3, and p300 immunostaining. The severity of glomerulosclerosis was expressed as the percentage of globally sclerosed glomeruli out of the total number of glomeruli in the renal biopsy. The nonglobally sclerosed glomeruli were analyzed for the presence of proliferative segmental lesions, crescents, micro-adhesions, and segmental (or global) sclerotic lesions. The degree of interstitial inflammation, interstitial fibrosis, and tubular atrophy was assigned a score between 0 and 3 according to the following: 0, 0–5%; 1, 6–20%; 2, 21–40%; and 3, >40% of the biopsy area demonstrating inflammation, fibrosis, or atrophy, respectively.
Statistical Analyses
Data are presented as mean±1 standard deviation. Comparisons between the groups were made with the Kruskal-Wallis test. Mann-Whitney U test was used to compare the molecules' expression between normal controls and every form of GN. The Spearman correlation analysis was used to detect possible correlations between staining scores at various nephron sites, clinical data, and indices of histologic injury. The association of pSmad2/3 with p300 expression in glomerular lesions of serial sections was assessed with χ2 test. Statistical significance was set at 5%.
RESULTS
Sp1 Expression in Normal Human Kidney
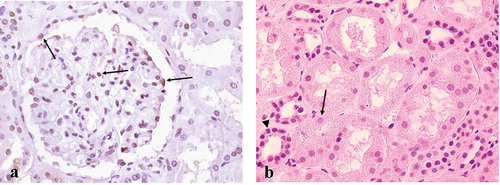
Within normal human kidney, about 40% of cells within the glomerulus were Sp1-positive, including podocytes, mesangial, endothelial, as well as parietal epithelial cells. Proximal tubules were negative or only very weakly stained, whereas distal and collecting tubules were constantly positive (see and b). The staining pattern was nuclear, as expected from immunohistochemical studies in other tissues.Citation[29]
Figure 2. Sp1 expression in normal human kidney. (a) About 40% of cells within the glomerulus were Sp1-positive, including podocytes, mesangial, endothelial, as well as parietal epithelial cells (arrows). (b) Proximal tubular cells (arrow) were either negative or weakly stained, while distal (arrowhead) and collecting tubules were constantly Sp1 positive. Original magnification ×400.
Sp1 Expression in Human Glomerulonephritis
Sp1 exhibited an increased glomerular and proximal tubular expression compared to controls in both proliferative and nonproliferative GNs (see and b). Proliferative glomerulopathies showed an increased Sp1 expression in proximal tubules (p = 0.025) compared to nonproliferative GNs (see ). Its expression was more intense in the glomeruli of the secondary group of proliferative GNs (p = 0.015) (see ). Compared to controls, no difference was detected in the expression of Sp1 in distal and collecting tubules of specimens with GN (see and d).
Figure 3. Glomerular (a), proximal tubular (b), distal tubular (c) and collecting tubular (d) staining scores of Sp1 in normal and diseased human kidneys. The error bars represent SE. *p < 0.05, **p < 0.01, ***p < 0.001. For abbreviations, see legend to .
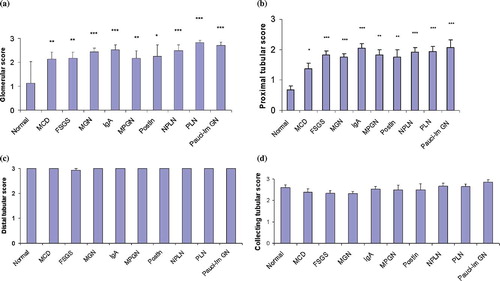
Table 2 Comparison of Sp1 staining intensity in proliferative vs. nonproliferative GNs
Table 3 Comparison of Sp1 staining intensity in secondary vs. primary proliferative GNs
Comparisons between the different types of GN with the Kruskal-Wallis test did not show a statistically significant difference in Sp1 expression in the glomeruli or tubules. With regard to pathologic lesions in the diseased glomeruli, Sp1 was almost constantly expressed in segmental hyperplastic lesions (100%) (see ), cellular and fibrocellular crescents (100%) (see ), and micro-adhesions (94%) (see ), while it was frequently detected in sclerotic glomeruli (83% each for globally and segmentally sclerosed glomeruli) (see and e and ). In cells overlying the sclerotic segments, Sp1 expression was prominent, whereas in serial sections, it seemed to co-localize with pSmad2/3 and p300 (see –).
Figure 4. Sp1 immunopositivity in various pathologic lesions of diseased glomeruli: a) hyperplastic lesions (proliferative lupus nephritis, WHO class IV), b) a cellular crescent (arrows) (Wegener granulomatosis), (c) a microadhesion (arrows) (proliferative lupus nephritis, WHO class IV), d) a globally sclerosed glomerulus, and e) a segmental sclerotic lesion (arrows) (IgA nephropathy); (e–g) serial sections of a glomerulus from a patient with IgA nephropathy exhibiting prominent Sp1 (4e), pSmad2/3 (4f) and p300 expression (4g) in cells overlying a sclerotic segment. (Figures 4e and 4g are reproduced from Kassimatis et al. [2006].Citation[17]) Original magnification ×400.
![Figure 4. Sp1 immunopositivity in various pathologic lesions of diseased glomeruli: a) hyperplastic lesions (proliferative lupus nephritis, WHO class IV), b) a cellular crescent (arrows) (Wegener granulomatosis), (c) a microadhesion (arrows) (proliferative lupus nephritis, WHO class IV), d) a globally sclerosed glomerulus, and e) a segmental sclerotic lesion (arrows) (IgA nephropathy); (e–g) serial sections of a glomerulus from a patient with IgA nephropathy exhibiting prominent Sp1 (4e), pSmad2/3 (4f) and p300 expression (4g) in cells overlying a sclerotic segment. (Figures 4e and 4g are reproduced from Kassimatis et al. [2006].Citation[17]) Original magnification ×400.](/cms/asset/438e07d5-bea4-4aab-860c-1f363f975d31/irnf_a_441294_f0004_b.jpg)
Table 4 The percentages of Sp1 immunopositivity incidence in the various lesions of the different groups of GN
Correlation of Sp1 with pSmad2/3 and p300 Expression at Corresponding Nephron Sites: Correlations between Immunohistochemical, Clinical, and Histologic Parameters
Taking into account all glomerulonephritides, the expression profile of Sp1 correlated positively with the expression of pSmad2/3 and p300 in proximal tubules (r = 0.241, p = 0.018 and r = 0.244, p = 0.014, respectively), while the same correlation stood for nonproliferative GNs (r = 0.367, p = 0.022 and r = 0.321, p = 0.024, respectively) (see ). In proliferative GNs, its expression correlated positively with pSmad2/3 expression in glomeruli (r = 0.32, p = 0.028).
Table 5 Correlation of Sp1 with pSmad2/3 and p300 expression at correspondent nephron sites
In all GNs, Sp1 glomerular and proximal tubular immunostaining correlated positively with serum creatinine levels (r = 0.265, p = 0.02 and r = 0.306, p = 0.006, respectively), while its proximal tubular expression showed a similar correlation with interstitial fibrosis (r = 0.213, p = 0.025). However, subgroup analysis revealed that the latter correlation held true only for nonproliferative GNs (r = 0.283, p = 0.042). No correlation was detected between Sp1 expression and 24-hour urinary protein, glomerulosclerosis, tubular atrophy, or interstitial inflammation (see ). No correlation was detected between Sp1 expression and lupus nephritis activity or chronicity indices, either.
Table 6 Correlations between immunohistochemical, clinical, and histologic parameters
DISCUSSION
In the present study, we sought to examine the expression of the transcription factor Sp1 in normal human kidney and in specimens from patients with various forms of GN. Correlations between Sp1 and pSmad2/3 and p300 expression were also made in order to detect possible associations of these molecules in mediating renal injury.
Sp1 was expressed in a significant number of all glomerular cell types as well as in distal and collecting tubules. This finding is in accordance with a previous study demonstrating that Sp1 was intensely expressed in mature rat glomeruli while its proximal tubular expression was weak.Citation[30] During nephrogenesis, Sp1 is temporally and spatially regulated according to Wilm's tumor-1 (WT-1) expression, suggesting that abundant Sp1 may be a prerequisite for WT-1 expression, and that Sp1 may have a wider role in nephrogenesis.Citation[30] Little WT-1 message is detected in undifferentiated metanephric mesenchyme,Citation[30–32] but levels increase prominently in the structures formed as the mesenchyme condenses, the renal vesicle, comma-shaped, and S-shaped body, where expression becomes localized to the developing glomerular podocytes.Citation[29] Therefore, Sp1 might play an important role in renal development (by regulating WT-1 expression) and in normal renal function.
This study provides the first demonstration of Sp1 overexpression in a broad spectrum of human glomerulonephritides. Sp1 was increased in the glomeruli and proximal tubules of GN patients compared to controls, being more prominent in the proximal tubules of proliferative GNs and in glomeruli of secondary proliferative GNs. Previous studies have shown that Sp1 directly regulates the expression of genes that are induced by TGF-β and are related to cellular proliferation, extracellular matrix degradation and angiogenesis. Sp1 overexpression has been demonstrated in a variety of cancers in which enhanced TGF-β signaling has been correlated with more aggressive phenotypes and poor prognosis.Citation[31,Citation32] Therefore, Sp1 may be implicated in the pathogenesis of hyperplasia that is observed in proliferative GNs either via mediating TGF-β or other signaling pathways. Moreover, the increased Sp1 expression in glomeruli of proliferative GNs and in proximal tubules of secondary proliferative GNs may be due to the increased inflammation that accompanies this disease process. It has been recently shown that Sp1 mediates MCP-1 expression in murine podocytes, which is implicated in the induction of renal injury through the attraction of macrophages.Citation[33] Moreover, Sp1 seems to regulate the transcription of other pro-inflammatory factors as well, thereby participating in tissue injury processes.Citation[34–36]
In segmental hyperplastic lesions as well as in cellular and fibrocellular crescents, which are hyperplastic lesions composed of epithelial cells and macrophages, Sp1 immunodetection was prominent. Based on the considerations made above, the increased Sp1 expression in these lesions might either imply a potential mitogenic influence of this factor in cells forming cellular crescents, or it may be due to its participation in signaling pathways of cytokines (such as TGF-β) that are excreted during the inflammatory crescent formation process.
Sp1 expression in micro-adhesions may imply a role of this molecule in the early stages of glomerulosclerosis. The co-localization of Sp1, pSmad2/3, and p300 in serial sections of glomeruli with micro-adhesions and segmental sclerotic lesions suggests a possible cooperation of these proteins in order to activate the transcription of genes responsible for glomerulosclerosis. As far as the chronic phase of glomerular injury is concerned, sclerotic glomeruli were almost constantly Sp1 and p300 positive, whereas pSmad2/3 immunostaining was frequently detected. This suggests a possible role for these molecules in advanced fibrotic events. This is the first study to demonstrate the co-localization of Sp1, p300, and pSmad2/3 in early and advanced sclerotic lesions.
The positive correlation of glomerular and proximal tubular Sp1 with the severity of renal dysfunction, along with the positive correlation of its proximal tubular expression with interstitial fibrosis, may imply a role of this molecule in the progression of renal disease. Moreover, the positive correlation of Sp1 with pSmad2/3 and p300 immunodetection in the diseased proximal tubules, as well as the positive correlation of pSmad2/3 and p300 co-expression in the diseased glomeruli and proximal tubulesCitation[17] and the common expression of these three molecules at sites of micro-adhesions, cellular crescents and segmental hyperplastic and sclerotic lesions, argue that these molecules cooperate to induce renal injury. Interestingly, it has been recently demonstrated that Smad proteins interact with Sp1 and p300 to mediate TGF-β signalling.Citation[26,Citation27]
In summary, this study has identified the expression of Sp1 in normal human kidney and a marked increase of its expression at various nephron sites in renal biopsies from patients with GN. Our findings suggest that Sp1 (probably through cooperation with pSmad2/3 and p300) plays an important role in the pathogenesis and the progression of human GN. Thus, the blockade of Sp1-pSmad2/3-p300 interaction or DNA binding inhibition of these complexes with the use of small molecules might provide for a novel strategy in the treatment of human GN. As these interactions seem to represent rather late steps in Smad signaling, these treatment strategies have the additional advantage of minimizing potential adverse effects by specifically blocking only the transcription of those genes that contribute to the fibrotic processes, without interfering with other parallel signaling pathways that are vital for other cellular functions.
ACKNOWLEDGMENTS
No authors have had any involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
REFERENCES
- Sporn MB, Roberts AB. Transforming growth factor-beta: Recent progress and new challenges. J Cell Biol. 1992;119:1017–1021.
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292.
- Border WA, Noble NA. TGF-beta in kidney fibrosis: A target for gene therapy. Kidney Int. 1997;51:1388–1396.
- Yamamoto T, Noble NA, Cohen AH, Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–469.
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791.
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347.
- Nakao A, Imamura T, Souchelnytskyi S, TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. Embo J. 1997;16:5353–5362.
- Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Adv Immunol. 2000;75:115–157.
- Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224.
- Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383:168–172.
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. Embo J. 1998;17:3091–3100.
- Zawel L, Dai JL, Buckhaults P, Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617.
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. Embo J. 2000;19:1745–1754.
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700.
- de Caestecker MP, Yahata T, Wang D, The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J Biol Chem. 2000;275:2115–2122.
- Shen X, Hu PP, Liberati NT, Datto MB, Frederick JP, Wang XF. TGF-beta-induced phosphorylation of Smad3 regulates its interaction with coactivator p300/CREB-binding protein. Mol Biol Cell. 1998;9:3309–3319.
- Kassimatis TI, Giannopoulou I, Koumoundourou D, Theodorakopoulou E, Varakis I, Nakopoulou L. Immunohistochemical evaluation of phosphorylated SMAD2/SMAD3 and the co-activator P300 in human glomerulonephritis: Correlation with renal injury. J Cell Mol Med. 2006;10:908–921.
- Kanamaru Y, Nakao A, Tanaka Y, Involvement of p300 in TGF-beta/Smad-pathway-mediated alpha2(I) collagen expression in mouse mesangial cells. Nephron Exp Nephrol. 2003;95:e36–e42.
- Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300.
- Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta-induced physical and functional interactions between Smads and Sp1. J Biol Chem. 2000;275:40014–40019.
- Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. J Biol Chem. 2001;276:6983–6992.
- Inagaki Y, Nemoto T, Nakao A, Interaction between GC box binding factors and Smad proteins modulates cell lineage-specific alpha 2(I) collagen gene transcription. J Biol Chem, 2001;276:16573–16579.
- Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem. 2000;275:39237–39245.
- Pardali K, Kurisaki A, Moren A, ten Dijke P., Kardassis D, Moustakas A. Role of Smad proteins and transcription factor Sp1 in p21(Waf1/Cip1) regulation by transforming growth factor-beta. J Biol Chem. 2000;275:29244–29256.
- Wu Y, Zhang X, Salmon M, Lin X, Zehner ZE. TGFbeta1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members. Biochim Biophys Acta. 2007;1773:427–439.
- Ihn H, Yamane K, Asano Y, Jinnin M, Tamaki K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatology (Oxford). 2006;45: 157–165.
- Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Tenascin-C upregulation by transforming growth factor-beta in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene. 2004;23:1656–1667.
- Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M. Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology. 1997;31:400–407.
- Wang L, Wei D, Huang S, Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–6380.
- Cohen HT, Bossone SA, Zhu G, McDonald GA, Sukhatme VP. Sp1 is a critical regulator of the Wilms' tumor-1 gene. J Biol Chem. 1997;272:2901–2913.
- Wang L, Guan X, Gong W, Altered expression of transcription factor Sp1 critically impacts the angiogenic phenotype of human gastric cancer. Clin Exp Metastasis. 2005;22:205–213.
- Abdelrahim M, Liu S, Safe S. Induction of endoplasmic reticulum-induced stress genes in Panc-1 pancreatic cancer cells is dependent on Sp proteins. J Biol Chem. 2005;280: 16508–16513.
- Gu L, Ni Z, Qian J, Tomino Y. Pravastatin inhibits carboxymethyllysine-induced monocyte chemoattractant protein 1 expression in podocytes via prevention of signalling events. Nephron Exp Nephrol. 2007;106:e1–e10.
- Chen G, Wang D, Vikramadithyan R, Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–4977.
- Chen Y, Currie RW. Heat shock treatment suppresses angiotensin II-induced SP-1 and AP-1 and stimulates Oct-1 DNA-binding activity in heart. Inflamm Res. 2005;54:338–343.
- Meissner M, Stein M, Urbich C, PPARalpha activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ Res. 2004;94:324–332.