Abstract
Aim. Aminoglycosides have been used in the treatment of CAPD peritonitis despite their potential risk for ototoxicity. The ototoxicity risk of intraperitoneally administered aminoglycosides has been investigated by a number of studies. However, their results are somewhat conflicting. The aim of the present study was to examine the frequency of hearing loss and the correlation between the repeated doses of aminoglycosides and hearing loss in CAPD peritonitis therapy. Methods. Hearing functions of the CAPD patients who had developed peritonitis and had been treated with various antibiotics including aminoglycosides were compared with those CAPD patients who had never developed peritonitis. Threshold values for hearing were determined through “pure tone audiometry” measurements. Results. Hearing threshold levels of the patients with history of peritonitis were found to be significantly higher in both lower [pure tone averages - 1 (PTA-1)] and higher [pure tone averages - 2 (PTA-2)] frequencies, when compared to the ones with no history of peritonitis (p values were 0.001 and 0.007, respectively). Conclusion. The present study showed that intraperitoneal aminoglycoside administration in CAPD patients is associated with the development of hearing loss. The severity of hearing loss may range from mild hearing loss to profound deafness. A remarkable correlation exists between the severity of the hearing loss and the repeated and total aminoglycoside dose received.
INTRODUCTION
Peritonitis currently continues to be one of the leading complications of continuous ambulatory peritoneal dialysis (CAPD).Citation[1–5] It is not only a reason for technical failure, but also leads to a considerable morbidity and mortality. Its incidence varies from center to center, and it resulted in death in 3% of the episodes in our cohort.Citation[6] Many patients are hospitalized due to peritonitis and may have to stop receiving CAPD therapy.Citation[7] According to the recommendations of the International Society for Peritoneal Dialysis, as soon as a patient is diagnosed with peritonitis, cultures must be taken and empirical antibiotics therapy should be started without delay.Citation[8–11] Empirical therapy has to cover both gram-positive and gram-negative microorganisms. Each PD center itself should determine antibiotics to be used in empirical therapy by being aware of the efficacies and sensitivities of the microorganisms causing peritonitis in its own patient population. Gram-positive organisms may be covered by vancomycin or a cephalosporin, and gram-negative organisms by a third-generation cephalosporin or aminoglycoside.Citation[11] Although aminoglycosides are recommended to be used for a short time because of the risk of ototoxicity,Citation[11] there are limited data regarding the correlation between the duration, dosage, and repeated usage of aminoglycosides, and the frequency of hearing loss. No certain correlation was established between intraperitoneal aminoglycoside administration and hearing loss in some of the previous studies.Citation[12–15] On the contrary, some studies demonstrate that the administration of intraperitoneal aminoglycosides causes ototoxicity, especially in repeated usage.Citation[16,Citation17] In most of the previous studies, the number of patients was limited and the correlations between hearing loss, cumulative dose, and the effect of repeated usage have not been investigated extensively. Our aim was to reveal the frequency and severity of hearing loss and the correlation between repeated aminoglycosides and/or vancomycin usages in peritonitis therapy for the patients with CAPD.
METHODS
This study was performed on the monitored patients with CAPD in Nephrology Department of the Medical Faculty of Erciyes University for a period of 24 months between June 2007 and June 2009. Patients over 18 years of age with end-stage renal disease (ESRD), who were on CAPD therapy for at least 12 months, had at least one peritonitis episode, and have been treated with cephalozine or vancomycin and/or amikacin were included in the study. All of the patients gave written informed consent. Forty-two patients with the same characteristics who had never suffered from peritonitis were used as a control group.
The patients with impaired tympanic membrane integrity were excluded. Each patient was queried in terms of complaints such as tinnitus, vertigo, and dizziness, and their responses were recorded.
Threshold values for hearing were identified through “pure tone audiometry” measurements with AC 40 Audiometer (Interacoustics, Denmark) using a standard technique of pure tone air and bone conduction thresholds measurement at frequencies of 250, 500, 1,000, 2,000, 3,000, 4,000, 6,000, 8,000, 12,000, and 16,000 Hz at Audiology Laboratory in the Otorhinolaryngology Department. Recordings of the threshold hearing values obtained from the ear with lower hearing were retained for analysis. The measured frequencies were separated into two groups and pure-tone averages (PTA) were gathered as follows:
Lower frequencies, PTA-1: 250, 500, 1,000, 2,000, 3,000, 4,000 and 6,000 Hz
Higher frequencies, PTA-2: 8,000, 12,000 and 16,000 Hz
The severity of hearing losses were categorized in .Citation[18]
Table 1 Categorization of hearing functions
Statistical Considerations
SPSS 11.0 software was used for the statistical analysis. The Kolmogorov-Smirnov test was used to determine the normality of distributions of variables. Continuous variables with normal distribution were presented as mean ± standard deviation. Where normal distribution was absent, the median value was used. The statistical analysis of the parametric variables was performed by the Student's t-test between two groups and by one-way ANOVA, with Scheffe's post-hoc test among more than two groups. The Mann-Whitney U test was used to compare nonparametric variables between the two groups. The Kruskal-Wallis test was used to compare nonparametric variables among more than two groups. Then, the Mann-Whitney U-test with Bonferroni correction was used to assess the differences among more than two groups. The correlation analysis was evaluated by the Pearson's correlation test for parametric variables and by the Spearman's correlation test for nonparametric variables. Qualitative variables were given as percentages, and the correlation between categorical variables was investigated by the chi-square test. The multivariate regression analysis was performed to determine the relationship between PTA-1, PTA-2 and the following variables: age, total amikacin dosage, total cephazolin dosage, total vancomycin dosage, episode of peritonitis, and serum albumin level. A p value of <0.05 was considered to be significant.
RESULTS
A total of 106 CAPD patients were included in the study. Forty-two of these patients never had peritonitis and served as a control group. The remaining 64 patients suffered from peritonitis for at least once and cephalozine, amikacin, and/or vancomycin had been used alone or together and in changing doses.
Demographic, clinical, and audiometric data for the patients with or without a history of peritonitis were summarized in . No statistical differences were found between the patients with or without a history of peritonitis in terms of age, gender, and ESRD etiology (p > 0.05). Residual urine volume and Kt/Vurea value of the patients without history of peritonitis were significantly higher (p = 0.001 and 0.018, respectively). The duration of dialysis was longer in the group with a history of peritonitis (p = 0.001). Complaints of dizziness, tinnitus, and vertigo were more frequent in the patients with a history of peritonitis (p = 0.001 for each).
Table 2 Demographic, biochemical, clinical, and audiometric parameters between patient groups with and without a history of peritonitis
Audio thresholds of the patients with a history of peritonitis were significantly higher in both lower (PTA-1) and higher (PTA-2) frequencies, compared with the patients without history of peritonitis (p = 0.001 and 0.007, respectively).
According to the severities of hearing loss, the distribution of the patients is given in . Based on the median hearing values for lower frequency sounds (PTA-1), it was found that 23.8% of the patients without history of peritonitis had mild hearing loss, while 64% of the patients with a history of peritonitis had moderate to severe hearing loss (p = 0.001). According to the median hearing values for higher frequency sounds (PTA-2), none of the patients with a history of peritonitis had normal hearing function; however, 23.4% of them had mild, 23.4% had moderate, 50% had severe, and 3.1% had profound hearing loss.
Table 3 Assessment of hearing impairment according to values of PTA-1 and 2
PTA-1 values were correlated with age (r = 0.329, p = 0.008), number of peritoneal attacks (r = 0.399, p = 0.001), total amikacin dose (r = 0.605, p = 0.001), and total vancomycin dose (r = 0.267, p = 0.033) (see ). On the other hand, PTA-1 values showed a reverse correlation with serum albumin level (r = -0.252, p = 0.044). However, no correlation was found with hemoglobin level, total cephalozine dose, duration of dialysis Kt/Vurea, 4-h D/P creatinine, and residual urine volume (p > 0.05).
Figure 1. Correlation between threshold hearing levels and usages of the increased doses of intraperitoneal cephalozine, vancomycin, and amikacin in CAPD peritonitis therapy.
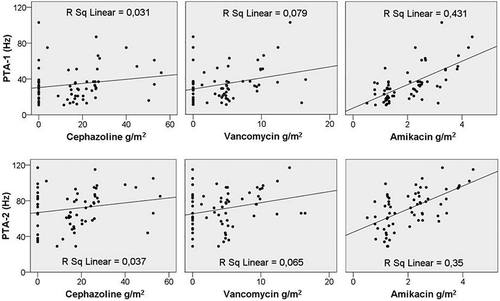
Similarly, PTA-2 values showed correlation with age (r = 0.414, p = 0.001), number of peritoneal attacks (r = 0.385, p = 0.002), total amikacin dose (r = 0.546, p = 0.001), and total vancomycin dose (r = 0.252, p = 0.045). However, no correlation was determined with total cephalozine dose, serum albumin and hemoglobin levels, residual urine volume, Kt/Vurea, duration of dialysis, and 4-h D/P creatinine ratio (p > 0.05).
The multivariate regression analysis, which was conducted to determine the correlation between PTA-1 and PTA-2 values and age, total amikacin dosage, total cephazolin dosage, total vancomycin dosage, episode of peritonitis, duration of CAPD, and serum albumin levels, showed that age and total amikacin dosage were found to be independent risk factors for both values of PTA-1 and PTA-2 (p = 0.009 and 0.001, respectively, for age; p = 0.001 and 0.001, respectively, for amikacin dosage).
DISCUSSION
Aminoglycosides have been used in the management of peritonitis in patients with CAPD despite a well-known ototoxicity risk. In spite of their side effects, the absence of safe alternatives to aminoglycosides is the main cause for using these antibiotics. On the other hand, aminoglycosides are highly advantageous drugs due to the fact that they are not expensive, bacterial resistance is lower, and allergic reactions are rare.Citation[19] In patients with CAPD, aminoglycosides are frequently used in peritonitis therapy through intraperitoneal route. The correlation between usage of intraperitoneal aminoglycosides and ototoxicity in the patients with CAPD was frequently investigated on the patients who were treated with tobramycin and gentamicin.Citation[12,Citation13,Citation15–17] On the other hand, it has been suggested that amikacin, another aminoglycoside, was more cochleotoxic than others.Citation[20]
In one of the early studies on this issue, Nikolaidis et al. suggested that the administration of intraperitoneal tobramycin in CAPD peritonitis did not lead to any increase in hearing loss.Citation[12] A possible reason for failure to determine the specific ototoxicity related to aminoglycosides in that study may be due to measurement of limited frequency range (i.e., between 250 and 10.000 Hz only).
Similarly, Gendeh et al. suggested that the therapy via intraperitoneal gentamicin does not increase ototoxicity risk by measuring serum drug levels in the patients with CAPD.Citation[13] However, it should be emphasized that only ordinary frequencies (128–8,000 Hz) were examined in that study, which might have resulted in not considering the patients who developed ototoxicity. In another study, the same investigators reported that the repeated intraperitoneal gentamicin administration increased the risk of ototoxicity.Citation[17]
Mars et al. reported that no hearing loss was developed in the study in which they performed audiogram during the starting of intraperitoneal aminoglycoside (within the first 24 hours administration) and after the completion of therapy (within 17 days in average) in the patients with CAPD.Citation[15] This study was conducted on only six patients, although it had a prospective design.
Another study performed on 19 patients with CAPD reported that clinical ototoxicity occurred even if gentamicin blood levels were not specifically increased after intraperitoneal administration.Citation[16] This study revealed that the hearing loss was observed especially in higher frequencies.
Our study showed that the patients with a history of peritonitis had significantly higher hearing loss according to the median hearing values for both lower and higher frequency sounds as compared to the patients with no history of peritonitis (p = 0.001 and 0.001, respectively). We found that the administration of aminoglycoside antibiotic to the patients with CAPD was directly associated with the development of the hearing loss. The hearing loss can be observed in degrees ranging from mild hearing loss to profound deafness. The obtained correlation is remarkable between the severity of the hearing loss and the repeated and total aminoglycoside dose received.
Gendeh et al. found that vancomycin might produce hearing impairment in CAPD patients when administered in combination with an aminoglycoside.Citation[21] It has been suggested that ototoxicity might be attributable to possible synergistic effect of two drugs.
Taking into consideration that the hearing loss can negatively affect the quality of life, the unnecessary repetitive usages of aminoglycoside antibiotics should be avoided, and the therapy should be stopped as soon as possible. The patients with CAPD who received ototoxic drugs should be followed up in terms of the complaints regarding hearing loss such as tinnitus, dizziness, or vertigo.
Of the mechanisms that are held responsible for aminoglycoside ototoxicity, the most frequently emphasized mechanism is that reactive oxygen types result in damage to internal ear.Citation[22] A recently published study showed that antioxidant therapy by using N-acetylcysteine was otoprotective in patients undergoing hemodialysis.Citation[23] Also, in patients with CAPD, studies investigating the protective effect of antioxidant therapy from ototoxicity are required.
Our study has some limitations. The most important is that the correlation between drug levels and hearing loss could not have been investigated, as no drug level measurement was performed for aminoglycoside. It will be useful to examine the issue in future studies including a prospectively efficient number of patients.
ACKNOWLEDGMENTS
The authors declare no conflicts of interests.
REFERENCES
- Fried LF, Bernardini J, Johnston JR, Piraino B. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol. 1996;7(10):2176–2182.
- Woodrow G, Turney JH, Brownjohn AM. Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int. 1997;17(4):360–364.
- Bayston R, Andrews M, Rigg K, Shelton A. Recurrent infection and catheter loss in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 1999;19(6):550–555.
- Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: Gram-negatives versus gram-positives in the Network 9 Peritonitis Study. Kidney Int. 1997;52(2):524–529.
- Piraino B, Bernardini J, Sorkin M. The influence of peritoneal catheter exit-site infections on peritonitis, tunnel infections, and catheter loss in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1986;8(6):436–440.
- Sipahioglu MH, Aybal A, Unal A, Tokgoz B, Oymak O, Utas C. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years' experience in a single center. Perit Dial Int. 2008;28(3):238–245.
- Piraino B, Bernardini J, Sorkin M. Catheter infections as a factor in the transfer of continuous ambulatory peritoneal dialysis patients to hemodialysis. Am J Kidney Dis. 1989;13(5):365–369.
- Keane WF, Everett ED, Golper TA, Peritoneal dialysis-related peritonitis treatment recommendations. 1993 update. The Ad Hoc Advisory Committee on Peritonitis Management. International Society for Peritoneal Dialysis. Perit Dial Int. 1993;13(1):14–28.
- Keane WF, Alexander SR, Bailie GR, Peritoneal dialysis-related peritonitis treatment recommendations: 1996 update. Perit Dial Int. 1996;16(6):557–573.
- Keane WF, Bailie GR, Boeschoten E, Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int. 2000;20(4):396–411.
- Piraino B, Bailie GR, Bernardini J, ; ISPD Ad Hoc Advisory Committee. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25(2):107–131.
- Nikolaidis P, Vas S, Lawson W, Is intraperitoneal tobramycin ototoxic in CAPD patients? Perit Dial Int. 1991;11(3):156–161.
- Gendeh BS, Said H, Gibb AG, Aziz NS, Zahir ZM. Gentamicin administration via peritoneal dialysis fluid: The risk of ototoxicity. Journal of Laryngology and Otology. 1991;105:999–1001.
- Nikolopoulos TP, Kandiloros DC, Segas JV, Auditory function in young patients with chronic renal failure. Clin Otolaryngol. 1997;22:222–225.
- Mars RL, Moles K, Pope K, Hargrove P. Use of bolus intraperitoneal aminoglycosides for treating peritonitis in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis and continuous cycling peritoneal dialysis. Adv Perit Dial. 2000;16:280–284.
- van der Hulst RJ, Boeschoten EW, Nielsen FW, Struijk DG, Drechler WD, Tange RA. Otototoxicity monitoring with ultra-high frequency audiometry in peritoneal dialysis patients treated with vancomycin or gentamicin. ORL J Otorhinolaryngol Related Spec. 1991;53(1):19–22.
- Gendeh BS, Said H, Gibb AG, Aziz NS, Kong N, Zahir ZM. Gentamicin ototoxicity in continuous ambulatory peritoneal dialysis. Journal of Laryngology and Otology. 1993;107:681–685.
- Clark JG. Uses and abuses of hearing loss classification. American Speech-Language-Hearing Association, ASHA. 1981;23(7):493–500.
- Gilbert D. Aminoglycosides. In Mandell GL, Bennet JE, Dolin R ( eds.). Principles and practice of infectious diseases. 6th ed. New York: Churchill Livingstone, 2005; 328–356.
- Selimoğlu E, Kalkandelen S, Erdoğan F. Comparative vestibulutoxicity of different aminoglycosides in the Guinea pigs. Yonsei Med J. 2003;44:517–522.
- Gendeh BS, Gibb AG, Aziz NS, Kong N, Zahir ZM. Vancomycin administration in continuous ambulatory peritoneal dialysis: The risk of ototoxicity. Otolaryngol Head Neck Surg.1998;118(4):551–558.
- Wu W, Sha S, Schacht J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol Neurootol. 2002;7:171–174.
- Feldman L, Efrati S, Eviatar E, Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007;72(3):359–363.