Abstract
Objectives. This study aimed to determine histopathologic changes in the pelviureteral junction in children with pelviureteral junction obstruction. Material and methods. Seventeen pelviureteral junction specimens obtained from children were divided into two groups: pelviureteral junction obstruction (n = 7) and control (n = 10). Wall thickness of the pelviureteral junction, tunica muscularis of the pelviureteral junction, uroepithelium thickness of the pelviureteral junction, and collagen thickness of the pelviureteral junction were evaluated in resected pelviureteral junctions in children with pelviureteral junction obstruction. Main findings. The mean wall thickness of the pelviureteral junction, mean tunica muscularis of the pelviureteral junction, and uroepithelium thickness of the pelviureteral junction were not significantly higher than those in the control group. Collagen thickness values in the pelviureteral junction obstruction group were significantly higher than those in the control group. Conclusions. Our data suggest that wall thickness (tunica muscularis and uroepithelium) was not significantly increased, but collagen thickness of the ureter was increased in the pelviureteral junctions of children with pelviureteral junction obstruction.
INTRODUCTION
Congenital obstructive nephropathy is the most frequent cause of renal failure in infants and children. Antenatal screening detects fetal hydronephrosis in 1 in 100 births, with about 20% clinically significant.Citation[1] Pelviureteral junction obstruction (PUJO) is found in 40–50% of these clinically significant cases, with an estimated incidence of 1 in 1000 to 1500.Citation[1] Currently, the adynamic dysplasia of this stenotic segment is considered the main pathologic change.Citation[2] Some investigators have reported abnormalities of smooth muscle or collagen in PUJO as revealed by light and electron microscopy. The abnormalities described include absent or deficient muscle at the PUJO, abnormal muscle orientation, and replacement of muscle by collagen.Citation[3–5]
Some investigators have suggested that defective histopathologic structures may play an important role in the pathogenesis of PUJO. However, little information is available about the histopathologic changes (HCs) in PUJO.Citation[6] These histopathologic changes include various factors such as wall thickness, collagen, and tunica muscularis in PUJO. Therefore, the first aim of this study was to test the hypothesis that tunica mucosa (uroepithelium) does not change in the stenotic tissue of patients with PUJO. We first examined the expression of uroepithelium in PUJO. The expression of various structural markers, including wall thickness, tunica muscularis, and collagen, was also examined in PUJO in our study.
We designed this study to investigate histopathologic changes, especially in the uroepithelium, in children with PUJO.
MATERIALS AND METHODS
Histological sections were analyzed from 10 patients that were operated on (by three pediatric surgeons with 16 years of experience) with radiologic findings of PUJO evaluated at the Meram Medical School between June 2006 and July 2008. All patients had been evaluated with standard radiological studies, including ultrasound, voiding cystourethrography, excretory urography, and diuretic renal scan. Material was obtained from children with PUJO (n = 10) in accordance with decision number 2006/113 of the Meram Medical School. Control specimens (n = 7) were obtained from children autopsied by one of the authors. The control group included five male and two female patients, 35 days to 12 years of age (mean 4.2 years). The patients who had renal pathology, frequent urinary tract infection (UTI), and urinary incontinence were not included in the control group after familial anamnesis was examined. In the study group, there were eight male and two female patients, 30 days to 10 years of age (mean five years). The patients in study group did not have crossing vessels as a contributing factor to UPJO, but did have symptoms including pain, mass, nausea, vomiting, and hypertension. The grade of PUJO in the study population was SFU (The Society of Fetal Urology) grade IV, which consists of significant calyceal dilatationCitation[7] before surgery, and the patients who were chosen to undergo surgery were included in this group. The analysis of the histological specimens was blinded to the pathologist.
For light microscopy, tissue specimens were fixed in 10% formaldehyde, processed in an autotechnicon, and embedded in paraffin. Sections of 4-μm thickness were cut with a microtome and stained with hematoxylin and eosin (H&E) and Tripple (Masson Trichrome, Merck) histochemically and Alpha Smooth Muscle Actin (α SMA) (Smooth Muscle Actin, DakoCytomation) immunohistochemically.Citation[8] The tunica muscularis of the pelviureteral junction (PUJ) was stained with α SMA. The differentiation of the muscularis and the fibrose tissue of the pelviureteral junction was performed using Tripple.Citation[9,Citation10]
Then, for each specimen, the same anatomic area after staining was photographed using a Nikon Coolpix 5000 photograph attachment. The Nikon micrometer microscope slide was also photographed during the procedure. All photographs were then transferred into the PC environment and analyzed using the Clemex Vision Lite 3.5 Image Analysis program. The length was calibrated by comparing the photograph of the specimen with the photograph of the Nikon micrometer microscope slide. Tubular epithelial cell length and tubular epithelial lumen were measured. The thickness of the PUJ, tunica muscularis, uroepithelium, and subepithelial collagen band of the PUJ part of the left kidney was measured in 10 different areas, and mean results were calculated automatically using Clemex Vision Lite 3.5.
All of the quantitative data were presented as mean ± SEM. The measurements were obtained in 10 different areas for every specimen, and the mean values of all specimens were identified. A Mann-Whitney test was used for wall thickness (tunica muscularis, uroepithelium) and collagen thickness values in comparisons of the two groups, and a chi-square test for inflammation values in comparisons of the groups. p values = .008 were considered statistically significant for the Mann-Whitney test; p values less than .05 were considered statistically significant for the chi-square test. In all computations, SPSS (version 15.00) was used.
RESULTS
The median wall thickness of the PUJ [886.7 (481.8–1287.0 μm], the median tunica muscularis of the pelviureteral junction [277.3 (173.9–502.0) μm], and the median uroepithelium of the pelviureteral junction [65.0 (29.4–126.4) μm] values of the PUJO group were not significantly higher than those [780.3 (498.3–1261.0), 317.2 (273.1–760.9), and 49.4 (28.1–80.4), respectively] in the C group (p >.05) (see and ). Collagen thickness [375.1 (207.7–667.0) μm] values in the PUJO group were significantly higher than those [99.3 (72.8–334.6)] in the C group (p =.008) (see and ).
Table 1 Histopathologic evaluation of the pelviureteral junction in humans [median (min–max)]
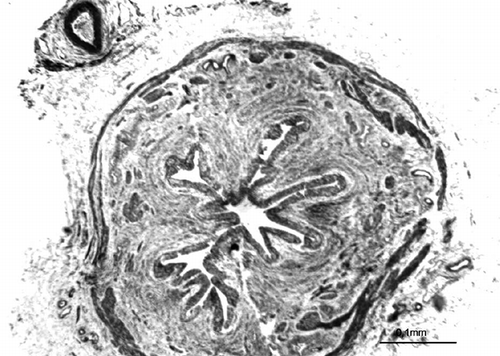
Figure 1. Photomicrograph of C group showing the morphology of a normal pelviureteral junction (Tripple).
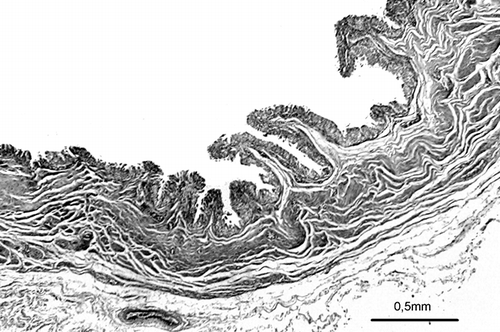
Figure 2. Photomicrograph of PUJO group showing the morphology of thick collagen in an obstructed pelviureteral junction (Tripple).
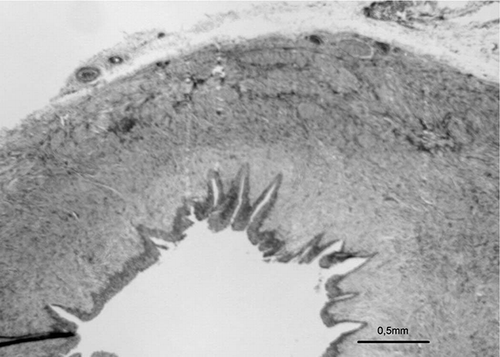
Figure 3. Photomicrograph of PUJO group showing the morphology of tunica muscularis in an obstructed pelviureteral junction (α SMA).
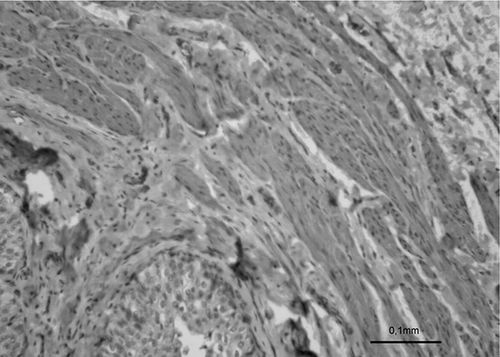
Figure 4. Photomicrograph of PUJO group showing the morphology of uroepithelium in an obstructed pelviureteral junction (H&E).
Inflammation in the PUJ in the PUJO group was greater compared to that in the C group according to the histopathological examination.
DISCUSSION
Pyeloureteral obstruction is a congenital obstruction to urine flow from the renal pelvis to the ureter. It is the most common cause of hydronephrosis in the fetal kidney. The pathophysiology of PUJO is still unknown but it appears to be multifactorial.Citation[3]
Most recent reports relating to congenital PUJO concentrate on the myogenic and neurogenic theories of peristalsis.Citation[11–16]
GoslingCitation[6] examined these obstructive segments and concluded that the muscle cells were normal, and that the excessive collagen fibers accounted for PUJO. Murakuma et al.Citation[17] proposed a combined neurogenic and myogenic theory. They concluded that in intrinsic obstruction, nerve fibers were depleted in the muscle layers in the ureteral walls, resulting in dysfunction and atrophy of the muscle fibers and an increase in collagen fibers in the muscle layers with abnormal accumulation of intercellular and interstitial collagen. In addition, Pinter et al. reported that the most consistent histological finding was hypotrophy of the smooth muscle in obstructing PUJ and its replacement with collagen.Citation[18] They suggested that these changes may disrupt the motility at the PUJ and lead to mechanical and functional obstruction.Citation[19] Zhang et al. reported that the PUJO area was consistently inflamed and markedly thickened due to varying degrees of perifascicular fibrosis and muscular hypertrophy,Citation[20] whereas Bartoli et al. reported that the stenotic tract showed partial or total loss of the epithelium and fibrosis of the mucosal and fibromuscular coats.Citation[21]
Previous publications have examined the structure and ultrastructure of the PUJO in childhood.Citation[6,Citation17,Citation19] A prior communication from our unit demonstrated for the first time that wall thickness (tunica muscularis and uroepithelium) was not significantly decreased, but collagen thickness of the ureter was decreased in the ureteral walls of children with vesicoureteral reflux.Citation[9] In our other study, diameter of the ureter lumen was identified to be significantly increased, but wall thickness, tunica muscularis, and epithelial thickness of the ureter were identified to be decreased in experimental congenital obstructive uropathy performed with adriamycin.Citation[10] Although this new information gave us a greater insight into the nature of dilated ureters, many questions remained. In an effort to further elucidate the pathologic nature of these PUJOs, we began to analyze our PUJO specimens for the structures in the PUJ.
As in other previous studies, the specimens in this study came from the PUJ in the kidney. They have clinical relevance because it is the complete PUJ that will be excised when these PUJOs are repaired.
The studies performed until now were on the myogenic and neurogenic theories of peristalsis, the muscle layers, and collagenCitation[11–18]; our study, on the other hand, was performed to compare the histology of wall thickness (tunica muscularis, and especially uroepithelium) and collagen thickness in the normal PUJ and PUJO in children. Although various findings regarding tunica muscularis and collagen were reported previously,Citation[6,Citation17,Citation18,Citation20,Citation21] we identified unchanged wall thickness (tunica muscularis and uroepithelium) and increased collagen in the subepithelial areas in the PUJ secondary to PUJO for the first time. The increased collagen thickness should be suspected as the cause of PUJ dysfunction in PUJO in childhood and may account for the decreased success rates of excision in these PUJs compared with those of normal-sized PUJs. If sufficient UPJ excision is not performed, the increased collagen in the PUJ prevents urine flow from the renal pelvis to the ureter.
Our study of the same PUJ segments in PUJO in comparison to controls clearly showed that there was extra collagen in the obstructed pelviureteral junctions, but the thickness of the wall, the overall muscle layer, and the uroepithelium were unchanged.
ACKNOWLEDGMENTS
We would like to thank Dr. Fatih Kara for his help with the statistical analysis. The authors declare no conflicts of interest.
REFERENCES
- Ransley PG, Dhillon HK, Gordon I, Duffy PG, Dillon MJ, Barratt TM. The postnatal management of hydronephrosis diagnosed by prenatal ultrasound. J Urol. 1990;144:584–587.
- Bernard MC, Waldo CF. Ureteropelvic junction anomalies: Congenital UPJ problems in children. In John PG, Richard C, Pierre DEM ( eds.). Pediatric urology. Philadelphia, WB Saunders; 2000;318–346.
- Williams B, Tareen B, Resnick MI. Pathophysiology and treatment of ureteropelvic junction obstruction. Current Urology Rep. 2007;8:111–117.
- Antonakopoulos GN, Fuggle WJ, Newman J, Considine J, O'Brien JM. Idiopathic hydronephrosis: Light microscopic features and pathogenesis. Arch Path Lab Med. 1985;109:1097–1101.
- D'Aquila RT, Bechtel LJ, Videler JA, Eron JJ, Gorczyca P, Kaplan JC. Maximizing sensitivity and specificity of PCR by pre-amplication heating. Nucleic Acids Res. 1991;19:3749.
- Gosling JA, Dixon JS. The structure of the normal and hydronephrotic upper urinary tract. In O'Reilly PH, Gosling JA ( eds.). Idiopathic hydronephrosis. Berlin, Springer-Verlag;1982:1–15.
- Gonzalez R, Schimke CM. Ureteropelvic junction obstruction in infants and children. In Sheldon CA, Churchill BM ( eds.). The pediatric clinics of North America (pediatric urology). Philadelphia, WB Saunders;2001;1505–1518.
- Rosai J, Ackerman S. Special techniques in surgical pathology. In Rosai J, Ackerman S ( eds.). Surgical pathology. Philadelphia, Mosby;2004;37–93.
- Yurtçu M, Gürbüzer N, Fındık S, Avunduk MC, Gunel EG. Investigation of histopathologic changes in the ureter walls in vesicoureteral reflux. J Pediatr Surg. 2009:44(4);802–805.
- Gürbüzer N, Yurtçu M, Fındık S, Avunduk MC, Gunel EG. Investigation of histopathologic changes in the ureter walls in experimental congenital obstructive uropathy. J Pediatr Surg. 2008;43(8):1524–1527.
- Knerr I, Dittrich K, Miller J, Kummer W, Rösch W, Weidner W, Rascher W. Alteration of neuronal and endothelial nitric oxide synthase and neuropeptide Y in congenital ureteropelvic junction obstruction. Urol Res. 2001;29:134–140.
- Hosgor M, Karaca I, Ulukus C, Ozer E, Ozkara E, Sam B, Ucan B, Kurtulus S, Karkiner A, Temir G. Structural changes of smooth muscle in congenital ureteropelvic junction obstruction. J Pediatr Surg. 2005;40:1632–1636.
- Solari V, Piotrowska AP, Puri P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol. 2003;170:2420–2422.
- Lama G, Ferraraccio F, Iaccarino F, Luongo I, Marte A, Rambaldi PF, Esposito-Salsano M. Pelviureteral junction obstruction: Correlation of renal cell apoptosis and differential renal function. J Urol. 2003;169:2335–2338.
- Yang Y, Zhou X, Gao H, Ji SJ, Wang C. The expression of epidermal growth factor and transforming growth factor-beta 1 in the stenotic tissue of congenital pelvi-ureteric junction obstruction in children. J Pediatr Surg. 2003;38:1656–1660.
- Demirbilek S, Edalı MN, Gurunluoglu K, Türkmen E, Taş E, Karaman A, Akin M, Aksoy RT, Celbis O, Uzün I. Glial cell line-derived neurotrophic factor and synaptophysin expression in pelviureteral junction obstruction. Urology. 2006;67:400–405.
- Murakumo M, Nonomura K, Yamashita T, Ushiki T, Abe K, Koyanagi T. Structural changes of collagen components and diminution of nerves in congenital ureteropelvic junction obstruction. J Urol. 1997;157:1963–1968.
- Pinter AB, Horvath A, Hrabovszky Z. The relationship of smooth muscle damage to age, severity of pre-operative hydronephrosis and post-operative outcome in obstructive uropathies. British Journal of Urology. 1997;80:227–233.
- Shafik A, Al-Sherif A. Ureteropelvic junction: A study of its anatomical structure and function—ureteropelvic junction sphincter? Eur Urol. 1999;36:150–157.
- Zhang PL, Peters CA, Rosen S. Ureteropelvic junction obstruction: Morphological and clinical studies. Pediatr Nephrol. 2000;14:820–826.
- Bartoli FA, Paradies G, Leggio A, Virgintino D, Bertossi M, Roncali L. Urothelium damage as the primary cause of ureteropelvic junction obstruction: A new hypothesis. Urol Res. 1996;24:9–13.