Abstract
A patient with idiopathic myelofibrosis with nephrotic syndrome is reported. Seven months after the initial diagnosis of myelofibrosis, the patient was presented with dyspnea, generalized edema, heavy proteinuria, massive pleural effusion, and ascites. Renal biopsy showed focal segmental glomerulosclerosis. After starting immunosuppressive therapy consisting of cyclosporine and steroids, his renal function and proteinuria improved and transfusion requirements decreased.
INTRODUCTION
Myelofibrosis with myeloid metaplasia (MMM) is a clonal hematopoietic stem cell disorder characterized by histological changes of the bone marrow, which include collagen fibrosis, osteosclerosis, and angiogenesis. Clinical features consist of constitutional symptoms, anemia, massive splenomegaly, extramedullary hematopoiesis (EMH), and an increase in hematopoietic precursors in the peripheral blood.Citation[1] It is thought that bone marrow stromal changes are reactive and caused by cytokines such as transforming growth factor beta-1 (TGF-β1), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), osteocalcin, epidermal growth factor, endothelial cell growth factor, interleukin-1, and osteoprotegerin (OPG) derived from clonal megakaryocytes and monocytes.Citation[2]
Renal abnormalities in idiopathic myelofibrosis have rarely been reported in literature, and include EMH resulting in obstructive uropathy, renal parenchymal hematopoietic cell infiltration, urolithiasis, acute renal failure as a result of hyperuricemia, nephrotic syndrome associated with mesangial proliferative glomerulonephritis, and amyloidosis.Citation[3,Citation4]
Here we present a patient with MMM who had nephrotic syndrome and whose renal biopsy revealed focal segmental glomerulosclerosis (FSGS).
CASE REPORT
A 50-year-old man was admitted to our hematology division in August 2005, with a six-month history of malaise, fatigue, pollakiuria, nocturia, and swelling of the legs and abdomen. His physical examination revealed splenomegaly of 10 cm and pretibial edema ++/++. Hemoglobin value was 7.3 g/dL, WBC was 3.6 × 109/L, MCV was 82 fl, and platelet count was 85 × 109/L. A blood smear showed leukoerythroblastosis, poikilocytosis, teardrop red cells, and polychromasia. Moreover, lactic dehydrogenase (LDH) level was elevated (1916 IU/L), and total protein and albumin levels were 5.5 g/dL and 2.8 g/dL, respectively. Erythrocyte sedimentation rate (ESR) was 105 mm/h. Urinalysis revealed microscopic hematuria and proteinuria, the latter being 1.12 g/day. Serum creatinine level was 70.8 μmol/L (0.8 mg/dL). An abdominal ultrasonography revealed a massive splenomegaly (201 mm), hepatomegaly (160 mm), and grade 1 renal parenchymal disease. Bone marrow aspiration and biopsy findings were consistent with myelofibrosis with myeloid metaplasia.
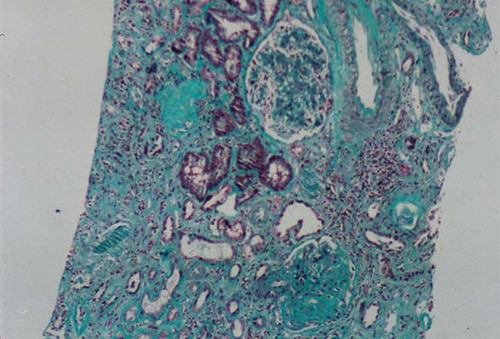
The patient was prescribed hydroxyurea 1000 mg/day and evaluated at nephrology outpatient clinic for hematuria and proteinuria. Because of thrombocytopenia, a renal biopsy could not be performed. An angiotensin-converting enzyme inhibitor was started to control his proteinuria. Two months later, he was hospitalized due to dyspnea, generalized edema, massive pleural effusion, and massive ascites. Complete blood count (CBC) and pertinent blood chemistry values were as follows: WBC, 2.9 × 109/L; Hb, 6.3 g/dL; MCV, 80 fl; PLT, 69 × 109/L; Cr, 185.85 μmol/L (2.1 mg/dL); total protein, 5.5 g/dL; serum albumin, 2.4 g/dL; LDH, 1908 IU/L; and daily urinary protein excretion, 14.5 g. Hepatitis serology was negative. We could not detect any amyloid deposits in rectal biopsy specimen and subcutaneous fatty aspiration. Because of leukopenia, hydroxyurea was stopped. A renal biopsy was performed. Light microscopic examination disclosed 13 glomeruli, 3 of which were globally sclerotic and 3–4 glomeruli disclosed segmental glomerulosclerosis. A single glomerulus contained hyalinosis. There were moderate tubular atrophy and interstitial fibrosis with mild patchy inflammation (see and ). Immunofluorescence staining of glomeruli revealed granular mesangial positivity for IgM. Staining for IgG, IgA, C3, C1q, and fibrinogen were negative. No immunofluorescence positivity was noted in tubular basement membranes or the interstitium. Congo red staining was negative in glomerular and tubulointerstitial compartments.
Figure 1. Light microscopic appearance of a renal biopsy demonstrated focal and segmental solidification of glomerular tuft, agglutination of capillaries with hyaline deposits. There is also interstitial infiltrate. H&E × 100.
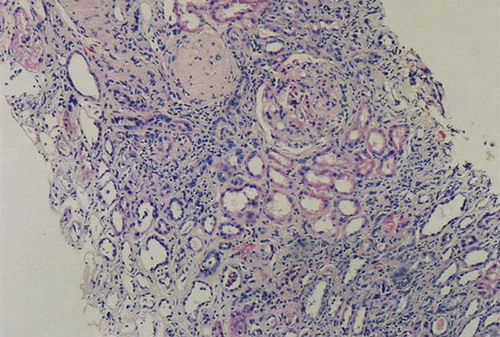
Figure 2. Light microscopic appearance of a renal biopsy showing intense fibrosis. Masson Trichrome staining × 200.
He was prescribed 500 mg of pulse I.V. steroid for three days and cyclosporine 200 mg/day. Two weeks later, because of increasing serum creatinine levels, hemodialysis was performed. In his follow-up, he did not require hemodialysis again. He was discharged with cyclosporine (200 mg/day) and methyl prednisolone (32 mg/day). Steroid dose was gradually tapered to 5 mg/day at three months. After one month of therapy, urinary protein excretion decreased to 3.34 g/day, his transfusion need ceased, and his renal function improved. He has been maintained on cyclosporine (150 mg/day, through level 78 ng/mL) and prednisolone 5 mg/day. Serum creatinine level was 194.7 μmol/L (2.2 mg/dL) at eight months after renal biopsy.
DISCUSSION
Myeloid metaplasia with myelofibrosis can affect many organ systems at the same time by different mechanisms. Our patient initially presented with pretibial edema, mild to moderate proteinuria, and hematological abnormalities. When he was diagnosed with MMM, he had accompanying mild to moderate proteinuria with normal renal function. He developed severe nephrotic syndrome with mild deterioration of renal functions six months after the initial diagnosis.
Renal abnormalities are rare in idiopathic myelofibrosis and consist of obstructive uropathy caused by EMH in the renal pelvis, ureters, bladder, renal parenchymal hematopoietic cell infiltration,Citation[5] urolithiasis, acute renal failure associated with hyperuricemia, and nephrotic syndrome with mesangial proliferative glomerulonephritis and membranous glomerulonephritis.Citation[3,Citation6] Amyloidosis complicating MMM was also reported in some case reports but was rare.Citation[4,Citation7,Citation8] Subcutaneous fat aspiration and rectal biopsy examination did not reveal amyloidosis. EMH, a characteristic feature of the disease, is most often found in the liver and spleen. The hematopoietic islands may be microscopic infiltrates or macroscopic tumors. EMH has also been reported in the kidneys.Citation[9] Hence, we performed a renal biopsy that demonstrated FSGS without EMH and amyloidosis.
FSGS refers to a common glomerular pathology that histologically reflects a final common pathway of various glomerular injury. FSGS has been reported as a consequence of hereditary/congenital disorders, vasculitis, metabolic disorders, human immunodeficiency virus infection, drug abuse, sickle cell disease, and others.Citation[10] FSGS has also been linked to a number of lymphoproliferative disorders.
Four cases of MMM associated with nephrotic syndrome secondary to glomerulonephritis have been reported.Citation[3,Citation6,Citation7,Citation11] In one study, 26 MMM patients were evaluated for renal abnormalities. One of the patients had glomerulonephritis with nephrotic syndrome.Citation[3] In three other cases, patients with MMM had nephrotic syndrome with renal EMH and mesangial proliferative glomerulonephritisCitation[6,Citation11] or membranous glomerulonephritis.Citation[7] To our knowledge, this is the first case of MMM associated with FSGS.
Significant clinical immunologic abnormalities including humoral and cellular immunity have been reported in MMM.Citation[12,Citation13] Clonality studies have shown that sometimes B and T cell lineages had clonal stem cell origins.Citation[2] In one study, T cell activation and elevation of plasma soluble interleukin-2 receptor levels have been reported in MMM patients.Citation[14] In addition, several reports have described some abnormalities of humoral immunity. Increased levels of circulating immune complexes have been found and their concentration correlated with the disease activity.Citation[13] Disorder of lymphocyte function, especially functional abnormalities of T lymphocytes, has been thought to be involved in the etiology of the primary FSGS through the production of factors that increase the permeability of the glomerular basement membranes.Citation[15] The pathogenetic mechanisms behind FSGS in our case were probably due to cytokines secreted locally or systematically. Previously, it was reported that PDGF, tumor necrosis growth factors, and IL-1β secreted from extramedullary hematopoietic foci resulted in interstitial and periglomerular fibrosis.Citation[5,Citation16–18]
After starting immunosuppressive therapy, his proteinuria decreased significantly to 1.3 g/day and renal function stabilized at 2.4 mg/dL of serum creatinine. Calcineurin inhibitors are effective in inducing remission in primary FSGS by hemodynamic as well as inhibiting IL-2 secretion and inhibition of T-cell proliferation.Citation[19,Citation20] No further blood transfusions were required, and platelet count was increased to normal levels. This hematological improvement might be related to potential therapeutic effects of immunosuppressives including steroids and/or calcineurin inhibitors.Citation[14,Citation21]
MMM is a hematopoietic disorder that might affect many organ systems and could be associated with different types of glomerular injury resulting in nephrotic syndrome. Patients should be followed carefully for other organ involvement Although our case suggests that FSGS could be a spectrum of glomerular injury accompanying MMM, further studies are warranted to elucidate the associations between MMM and FSGS.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265.
- Mesa R, Barosi G, Cervantes F. Myelofibrosis with myeloid metaplasia: Disease overview and non-transplant treatment options. Best Practice & Research Clinical Hematology. 2006;19:495–517.
- Caijo Y, Almange C, Boutin J, Leblay R, Guerin D. Renal lesions in myelofibrosis. Sem Hop. 979;55:1271–1274.
- Ferhanoglu B, Erzin Y, Başlar Z, Tuzuner HA. Secondary amyloidosis in the course of idiopathic myelofibrosis. Leukemia Research. 1997;21:897–898.
- Oesterling JE, Keating JP, Leroy AJ, Idiopathic myelofibrosis with myeloid metaplasia involving the renal pelvis, ureters and bladder. J Urol. 1992;147:1360.
- Liu TT, Chen JB, Chen WJ, Kuo CY, Lee CT. Idiopathic myelofibrosis associated with renal extramedullary hematopoiesis and nephrotic syndrome: Case report. Chang Gung Med J. 2000;23:169–174.
- Pamuk ON, Pamuk GE, Altiparmak MR, Sonsuz A, Solakoglu S, Kilicaslan I. Nephrotic syndrome associated with agnogenic myeloid metaplasia. Leuk Lymphoma. 2002;43: 661–663.
- Chan KW, Ho CP. Amyloidosis complicating idiopathic myelofibrosis. Am J Kidney Dis. 1999;34:E27.
- Kwak H, Lee J. CT findings of extramedullary hematopoiesis in the thorax, liver and kidneys in a patient with idiopathic myelofibrosis. J Korean Med Sci. 2000;15:460–462.
- Cameron JS. Focal segmental glomerulosclerosis in adults. Nephrol Dial Transplant. 2003;18(Suppl. 6):45–51.
- Perazella MA, Buller GK. Nephrotic syndrome associated with agnogenic myeloid metaplasia. Am J Nephrol. 1994;14: 223–225.
- Gordon BR, Coleman M, Kohen P. Immunologic abnormalities in myelofibrosis with activation of the complement system. Blood. 1981;58:904–910.
- Vellenga E, Mulder N. A study of the cellular and humoral immune response in patients with myelofibrosis. Clin Lab Heamatol. 1982;4:307–316.
- Centenara E, Guarnone R, Ippoliti G, Barosi G. Cyclosporine-A in severe refractory anemia of myelofibrosis with myeloid metaplasia: A preliminary report. Hematologica. 1998;83: 622–626.
- Savin VJ, Sharma R, Sharma M, Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883.
- Schnuelle P, Waldherr R, Lehmann KJ, Idiopathic myelofibrosis with Extramedullary hematopoiesis in the kidneys. Clin Nephrology. 1999;52:256–262.
- Abboud HE. Role of platelet-derived growth factor in renal injury. Annu Rev Physiol. 1995;57:297–309.
- Sterzel RB, Hartner A. Schlotzer-Schrehardt U, et al. Elastic fiber proteins in the glomerular mesangium in vivo and in cell culture. Kidney Int. 2000;58:588–602.
- Daniel V, Trautmann Y, Konrad M, Nayir A, Schärer K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997;47:289–297.
- Cattran DC, Appel GB, Hebert LA, A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226.
- Pietrasanta D, Clavio M, Vallebella E, Beltrami G, Cavaliere M, Gobbi M. Long-lasting effect of cyclosporine-A on anemia associated with idiopathic myelofibrosis. Hematologica. 1997;82:458–459