Abstract
Cisplatin (CP) nephrotoxicity is mainly due to reactive oxygen species. Oxygen pre-exposure as a mild oxidative stress may enhance some endogenous defense mechanisms, so its effect on cisplatin-induced acute renal failure was investigated in present study. Twenty-four rats were divided into four groups. The O2+ CP and Air + CP groups were were subjected to i.p. injection of 5 mg/kg cisplatin, and in the Air + Saline and O2 + Saline groups, saline was injected instead of cisplatin. O2+ CP and O2+ Saline groups were pretreated with oxygen (3h/d for two days), and the other two groups were pretreated with room air. Cisplatin was administered 24 h after last pretreatment session. Three days after cisplatin injection, plasma samples were obtained, and parts of kidney tissue were frozen for biochemical analysis or fixed in formalin for histological assessments. Preconditioning with oxygen prior to cisplatin administration led to reduced tubular necrosis and luminal cast formation and improvement of renal function, as was evidenced by significant reduction in plasma creatinine and urea levels. Oxygen pretreatment also significantly reversed cisplatin-induced reduction in renal catalase activity and glutathione level. It could be concluded that oxygen pretreatment could have a delayed protective effect against cisplatin nephrotoxicity, and that increased renal catalase activity may be involved in this protective effect of hyperoxia.
INTRODUCTION
Cisplatin (CP) is a major antineoplastic drug for treatment of various solid tumors,Citation[1] but its nephrotoxicity is a considerable dose-limiting side effectCitation[2] and is the chief cause of acute renal failure in about 20% of hospitalized patients.Citation[3] Indeed, irreversible renal damage occurs in about one-third of cisplatin-treated patients despite using known prophylactic methods like hydration.Citation[4,Citation5]
There is almost a century of literature that supports the concept that prior injury may protect the kidney against a second insult, and it has been shown more recently that the primary stimulus may not necessarily reach the injurious level.Citation[6] For example, sub-lethal short ischemic periods may attenuate subsequent renal functional or structural damage induced by prolonged ischemia.Citation[7,Citation8] We have shown recently that intermittent pretreatment of rats with nearly pure oxygen for durations far beyond the oxygen toxicity threshold could lead to some degree of renal protection against ischemia-reperfusion injury.Citation[9] Cisplatin causes significant oxidant loading to the kidney through both free radical formation and impaired antioxidant defense systems.Citation[10]
The hypothesis underlying the present study is that mild oxidative stress induced by oxygen pre-exposureCitation[11] may enhance some endogenous defense mechanisms such as renal antioxidant systems and reduce subsequent cisplatin-induced kidney damage resulting from reactive oxygen species. Keeping this in view, we compared the degree of renal functional and structural injury in rats that were subjected to high-dose cisplatin administration with or without oxygen pretreatment. In addition, renal superoxide dismutase (SOD) and catalase (CAT) activities and glutathione (GSH) and malondialdehyde (MDA) levels were measured. It should be noted that short-term pre-exposure to hyperoxic environment is a benign protocol that could easily be used in the clinical practice.Citation[12]
MATERIALS AND METHODS
Animals and Oxygen Exposure
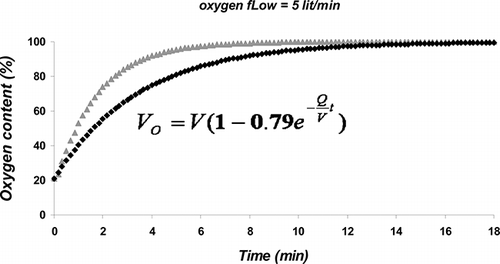
The experimental protocol used in this study was approved by the Baqiyatallah University of Medical Sciences Ethics Committee for Animal Research. Adult male Wistar rats (251.3 ± 6.3 g) were obtained from Tehran University of Medical Sciences animal house and had free access to rodent food and water. Oxygen exposure in two groups of animals was achieved by placing them in a specific chamber (35 × 25 × 20 cm). Normal air was removed from the chamber for about 10 min and replaced by pure oxygen delivered at ≥5 lit/min. Thereafter, a 1–2 lit/min oxygen inflow was maintained to produce a stabilized hyperoxic environment which contained ≥95% oxygen without CO2 retention. Animals of the other two groups were kept in a similar chamber but with a continuous delivery of room air by means of an aquarium air pump. Oxygen content of the chamber was measured continuously by an oxygen meter (Lutron DO-5510, Taiwan). A mathematical formula was equated for predicting the oxygen content in the chamber (see ). In three anesthetized rats, the right common carotid artery was cannulated, and at the end of 3 h oxygen exposure, a blood sample was withdrawn to estimate the arterial oxygen partial pressure (PaO2). The values for PaO2 in these three rats were 404, 435, and 468 mmHg. In three other rats, PaO2 values were 96, 89, and 95 mmHg after 3 h exposure to room air in the same chamber. (The difference between PaO2 values in these two groups of rats was statistically significant; p = 0.05, Mann-Whitney U test.)
Figure 1. Percent of oxygen gas in the chamber. There was a high degree of correlation (p < 0.001, R2 = 0.93) between experimental data (gray triangles) and data predicted by a mathematic formula, which was equated for this purpose (black squares). It has been presupposed in this equation that the oxygen source has 100% purity and that the initial O2% in the chamber is 21%. It is obvious from equation that mathO2% = VO/V = 1 - 0.79e–Qt/V. According to the correlation, the O2 content of chamber can be equated from mathO2%: O2% = 0.95 (mathO2%) + 15. These equations can be used when there is no access to oxygen meter or for designing new chambers with different volumes (e.g. for human subjects). Abbreviations: mathO2% = O2% predicted by the mathematical formula, VO = volume of oxygen gas in the chamber (lit), V = chamber volume (lit), Q = oxygen flow (lit/min), t = time (min) (i.e., the period of oxygen inflow).
Experimental Design
In a pilot study, the effects of 0.5, 1, 3, and 6 h and 3 h/d for two days of pretreatment with nearly pure oxygen (≥95%) on cisplatin-induced nephropathy was investigated, and a 3 h/d exposure to hyperoxia for two days was found to have the most protective effects. Thereafter, 24 rats were divided randomly into four groups. The O2+CP and Air+CP groups received an i.p. injection of 5 mg/kg cisplatin (1 ml per 100 g body weight from 50 mg/100 ml solution; Ebewe Pharma, Austria). The cisplatin dosage for nephrotoxicity induction was like some other studies.Citation[13,Citation14] In the Air+Saline and O2+Saline groups, normal saline (1 ml per 100 g body weight) was injected instead of cisplatin. The O2+CP and O2+Saline groups were pretreated with oxygen 3 h/d for two days. The other two groups were kept in the same chamber for similar lengths of time but with an inflow of normal air. The time of cisplatin injection was 24 h after their removal from the chamber. Three days after cisplatin injection, the animals were anesthetized with an i.p. injection of sodium pentobarbital (Sigma Chemical Co; 50 mg/kg) and the left kidney was removed after laparotomy. Blood samples were obtained from the aorta. Plasma samples were kept in (−24°C) until analysis. The rats were weighed both before and three days after cisplatin administration.
Assessment of Renal Function
Plasma urea was measured using Berthelot procedure (ZistChem kit, Iran), and plasma creatinine (Cr) was measured using Jaffe reaction (Pars Azmoon kit, Iran) by an autoanalyzer (Hitachi 902, Japan).
Assessment of Plasma Magnesium
Plasma magnesium (Mg) was determined utilizing Xylidynblue method (Pars Azmoon kit, Iran).
Tissue Preparation
The kidneys were bisected along the long axis, and each half was cut into two equal pieces. Two pieces were frozen rapidly using liquid nitrogen and kept at −70°C until used. For making estimations, the frozen tissue samples were quickly weighed and homogenized in 1:10 ice-cold 50 mM potassium phosphate buffer (pH 7.4) containing 1 mM EDTA and proteases inhibitors. The homogenates were then centrifuged at 12,000 g for 15 min at 4°C. The supernatants were taken and used for enzyme, MDA, GSH, and protein assay.
Catalase and SOD Activities Assay
Catalase activity in kidney tissue homogenates was measured by a colorimetric method as described previously by Cohen.Citation[15] The SOD activity was determined according to Paoletti method.Citation[16]
GSH and MDA Determination
GSH level was determined by the method of Tietz.Citation[17] Plasma and tissue malondialdehyde (MDA) levels as markers of lipid peroxidation were quantified according to Satoh procedure.Citation[18]
Protein Assay
Total protein concentration was measured by Bradford's methodCitation[19] using bovine serum albumin as standard.
Histological Assessments
Kidney slices were fixed in 10% formalin solution and embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin. Histological assessment was performed by two experienced pathologists who were completely unaware of the animal groupings. Degree of tubular necrosis and proteins cast formation were graded from 0 to 4 (0 = normal, 1 = marginal, 2 = mild, 3 = moderate, and 4 = severe). Congestion and/or hemorrhage were graded in a manner similar to Solez et al.Citation[20]: 0 = no congestion and/or hemorrhage, +1 = identification of erythrocytes by 400× magnification, +2 = identification of erythrocytes by 200× magnification, +3 = identification of erythrocytes by 100× magnification, and +4 = identification of erythrocytes by 40× magnification.
Statistical Analysis
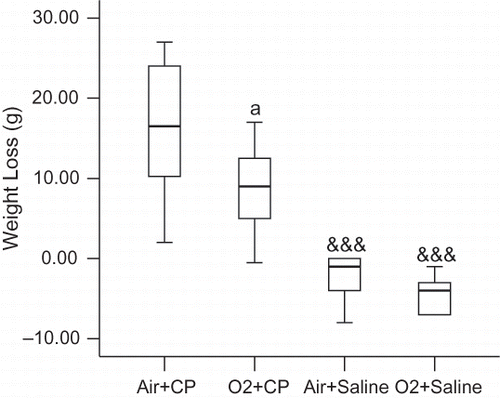
Data were expressed as median (range). SPSS 13 software was used for statistical analysis and comparisons were performed using Mann-Whitney U test. The significance of weight loss or gain was analyzed in each group by Wilcoxon test. p ≤ 0.05 was considered significant.
RESULTS
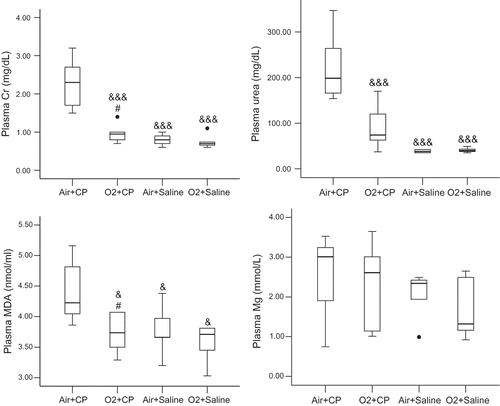
Preconditioning with oxygen led to significant reduction in plasma creatinine, urea, and MDA levels in O2 + CP group compared to Air + CP group (see ). Plasma Mg levels did not vary significantly between the two groups (see ).
Figure 2. Plasma creatinine (Cr), urea, malondialdehyde (MDA), and magnesium (Mg) levels in various experimental groups. O2 + CP and O2 + Saline groups were exposed to oxygen (≥95%) 3 h/day for two days. O2 + CP and Air + CP groups received an i.p. injection of 5 mg/kg cisplatin. Rats in the Saline groups received normal saline instead of cisplatin, and Air groups were placed in the same chamber with continuous flow of room air via an aquarium pump. The time of cisplatin injection was 24 hours after exit from the chamber. Plasma samples (also tissue samples) were obtained three days after cisplatin injection. There was no significant difference between the two saline-treated groups. •an out of range data; &p < 0.05; &&&p < 0.005 in comparison with Air+CP group; #not significantly different from O2 + Saline group (also not significantly different from Air + Saline group).
The histological assessment of kidneys from the Air + CP group showed the glomeruli to be normal. Many tubules showed necrotic changes in epithelial linings, most of which were proximal ones in the lower cortex and outer medulla areas with a predominance of S3 segment. Necrotic cells showed karyorrhexis and rupture of cytoplasmic membrane. Some tubules were completely denuded of epithelial cells. There were also necrotic changes in a few distal tubules, some of which were located adjacent to the glomerular stalk. Hyaline casts were present in both cortical and medullary areas. These abnormal changes were absent in the Air + Saline and O2 + saline groups and less prominent in the O2 + CP group (see and ). Vascular congestion and hemorrhage were more prominent in the Air + CP group compared to the O2 + CP group (see ). Interstitial edema and lymphocytic infiltration were not remarkable in any group.
Figure 3. Light microscopy photographs of kidney specimens (H&E, ×400) from Air + Saline (A), O2 + Saline (B), Air + CP (C), and O2 + CP (D) groups. For a brief explanation of groups, see . A and B are normal. In C, which is from a case with severe tubular necrosis, there are some necrotic tubules (arrows). In D, which is from a case with mild tubular necrosis, there is only one necrotic tubule (arrow). There were many fields with normal appearance in this case, which was preconditioned by oxygen administration 24 hours before cisplatin injection.
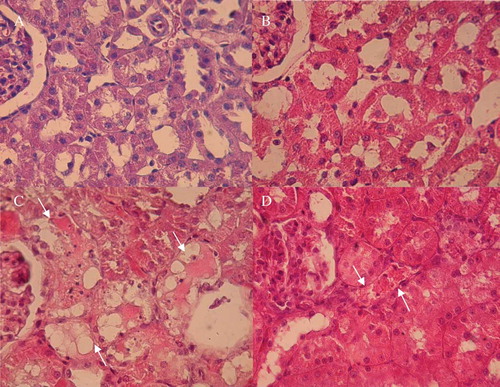
Table 1 Degree of tubular necrosis, luminal cast formation, and congestion and/or hemorrhage in microscopic examination of kidney specimens from different groups
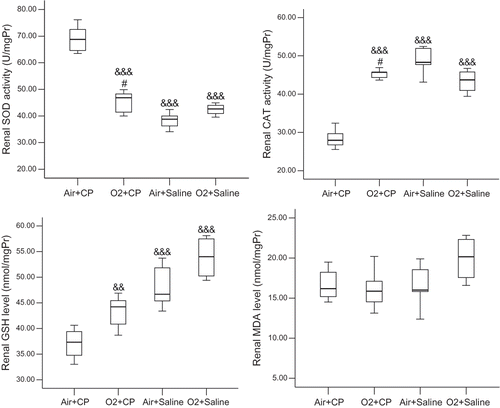
Cisplatin administration in the absence of oxygen pretreatment led to a significant increase in SOD activity and reduction in catalase activity and GSH level. Renal SOD activity in the O2 + CP group was significantly lower than in the Air + CP group but not different from the O2 + Saline group. Renal catalase activity was higher in the O2 + CP group than in the Air + CP group. The difference in catalase activity was not significant between the O2 + CP group and groups receiving saline instead of cisplatin. Also, oxygen pretreatment resulted in renal GSH level elevation in the O2 + CP compared to the Air + CP group. Renal SOD and catalase activities and GSH levels were not significantly different between the two saline-receiving groups. There was no significant difference in renal MDA levels in the experimental groups (see ).
Figure 4. Renal superoxide dismutase (SOD) and catalase (CAT) activities and glutathione (GSH) and malondialdehyde (MDA) levels. For a brief explanation of groups, see . There was no significant difference between the two saline-treated groups. &&p < 0.01; &&&p < 0.005 in comparison with Air + CP group; #not significantly different from O2 + Saline group.
Weight loss or gain data in various groups are shown in .
DISCUSSION
The major finding of this study is that oxygen pretreatment exerts a considerable protective effect against cisplatin-induced nephrotoxicity in rats. This protective effect, which was present 24 hours after oxygen administration, was evidenced both in renal structure and function. It seems that this is the first study that indicates this effect of oxygen pretreatment on renal tissue. Oxygen may exert at least some part of this protective effect by the induction of catalase activity.
Oxygen-derived free radicals have an important role in cisplatin-induced acute renal failure (ARF).Citation[21] Many antioxidant agents like vitamin E or C have been reported to either ameliorate or prevent the cisplatin nephrotoxicity;Citation[22] among them is N-acetylcysteine, which has been reported to reduce cisplatin-induced nephrotoxicity in both animals and humans.Citation[23–25] The protective effect of some plant extracts on cisplatin nephrotoxicity has been associated with an enhancement of renal antioxidant defense systems.Citation[26]
In contrast to some other studies,Citation[26] cisplatin administration resulted in increased SOD activity in our study. It should be noted that an increased SOD expression in the organism cannot always be regarded as a positive effect. Hydrogen peroxide (H2O2) produced by catalytic effect of SOD is probably more harmful than superoxide. Furthermore, hydroxyl radical, which is produced in large quantities from H2O2 in the absence of enough catalase activity, is a very reactive and dangerous radical.Citation[27] Thus, like increased catalase activity, the reduction of SOD activity in the O2 + CP group to almost normal levels could have some protective features, as reported for other tissues after normobaric oxygen administration.Citation[28]
Free radicals have a major role in ischemia-reperfusion (IR) injury of various organs, and thus the mechanisms responsible for the reduction of IR injury may also work against cisplatin-induced free radical injury. It has been shown that pre-exposure to normobaric hyperoxia could be protective against subsequent brain,Citation[29] heart,Citation[30] spinal cord,Citation[31] and renal IR injury.Citation[9]
Atasoyu et al. concluded that the synchronous application of HBO therapy (60 min every day for seven days at 2.5 ATA) with cisplatin prevents kidney damage by decreasing necrosis scores.Citation[32] In our study, only two sessions of normobaric oxygen administration were used 24 h before cisplatin injection. It seems that the use of oxygen prior to cisplatin administration could have a considerable effect. Oxygen therapy by itself seems to induce an excess ROS formationCitation[33] and may promote the ROS-induced injury of cisplatin, thereby reducing the protective effect of oxygen. Therefore, it is not surprising that in Aydinoz's study, daily HBO therapy (for six days) reduced kidney injury but twice daily HBO usage considerably augmented cisplatin-induced ARF.Citation[34] It should be noted that both in Atasoyu and Aydinoz studies, intermittent HBO therapy was started after the cisplatin injection. It could be proposed that short duration pretreatment with oxygen leads to a mild oxidative stress,Citation[11] and this sub-lethal oxidative stress may in turn enhance endogenous defense mechanisms, such as antioxidant molecules and enzymes, against cisplatin-induced free radical injury.
Cisplatin leads to anorexia,Citation[35] and this could be the reason underlying weight loss following its administration.
There are surprising differences in various studies about the effect of cisplatin on renal MDA content (i.e., from no significant changeCitation[36] as reported in our study to dramatic increases in cisplatin treated animalsCitation[37]). It seems that the results arising from the various spectrophotometric methods of MDA assay are different and not very precise (the HPLC may be more accurate).Citation[38] Decreased plasma MDA level in the O2 + CP group compared with the Air + CP group despite insignificant differences in renal MDA levels between these two groups may be related to less overall lipid peroxidation in the O2 pretreated group because of the up-regulation of the defense mechanism in tissues other than kidney. The degree of changes in plasma MDA levels at different time intervals after cisplatin administration remains to be investigated.
Although cisplatin can lead to hypomagnesemia, there was no difference in the plasma Mg levels among the various groups in this study. It appears, as described earlier,Citation[39] that this effect of cisplatin on plasma Mg level becomes apparent at least seven days after cisplatin administration. Thus, it is not surprising that three days after the cisplatin injection, there was no difference in plasma Mg levels between the different groups.
A question remains to be answered: might this beneficial effect of oxygen administration be associated with the attenuation of antineoplastic activity of cisplatin? Indeed, there is some evidence that suggests that oxygen does not reduce the therapeutic effects of cisplatin. For example, in Teicher et al.’s elegant study, cisplatin has been classified as a drug in which the degree of cell oxygenation has no influence on its therapeutic effects.Citation[40] Daily HBO administration in mice not only does not reduce the therapeutic effect of weekly cisplatin on the bulky hypoxic tumors of epithelial ovarian cancer, but it also enhances the response to chemotherapy, which is likely due to increased tumoral vascularity.Citation[41] Similarly, it has been determined that O(2/3) gas mixture has a direct anti-tumoral effect on human neuroblastoma cell lines, and that the combined O(2/3) and cisplatin treatment produces a stronger cell inhibitory effect compared to when cisplatin is used alone, which is attributed to the cell growth inhibition effect of oxygen therapy.Citation[42] Nevertheless, it seems that the exact effect of oxygen pretreatment on therapeutic effects of chemotherapeutic agents has not been studied comprehensively and needs to be done.
Finally, it should be stated that pure oxygen at atmospheric pressure is safe for clinical application if given for less than six hours,Citation[12] and the first morphologic changes in rat lungs exposed to 100% oxygen appear only after 40 h of continuous exposure,Citation[43] far beyond the 3 h exposure used in the present study.
In conclusion, short duration pre-exposure to oxygen could reduce the nephrotoxicity resulting from single-dose cisplatin administration, and it is suggested that increased renal catalase activity and glutathione levels may be involved in this protective effect of hyperoxia. The effect of oxygen pretreatment on weekly cisplatin treatment, its influence on antitumoral effects of cisplatin, and some other commonly used chemotherapeutic agents requires more extensive studies.
ACKNOWLEDGMENTS
The authors would like to thank Professor Ashima Anand for English revision and Mr. Saeed Otadi, MSc, Dr. Alireza Asgari, Dr. Hamidreza Dehghan Manshadi, Mrs. Safieh Otadi, Mrs. Maryam Tabatabaei, Mrs. Parisa Rasoulian, Dr. Hedayat Sahraei, Dr. Mehri Kadkhodaee, and Mr. Ali Noroozzadeh, MSc, for their kind help and comments.
Financial support by Lorestan University of Medical Sciences and Baqiyatallah University of Medical Sciences is gratefully acknowledged.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Livingston RB. Cisplatin in the treatment of solid tumors: Effect of dose and schedule. J Natl Cancer Inst. 1989 May 10;81(10):724–725.
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003 Sep;23(5):460–464.
- Berns JS, Ford PA. Renal toxicities of antineoplastic drugs and bone marrow transplantation. Semin Nephrol. 1997 Jan;17(1):54–66.
- Santoso JT, Lucci JA III, Coleman RL, Schafer I, Hannigan EV. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: A randomized trial. Cancer Chemother Pharmacol. 2003 Jul;52(1):13–18.
- Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107–121.
- Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002 Jan;11(1):43–48.
- Mohammadhosseiniakbari H, Rasoulian B, Noroozzadeh A, The effect of short ischemic periods in reducing subsequent rat renal ischemic injury. Physiology and Pharmacology. 2008;12(2):149–157 [in Persian].
- Torras J, Herrero-Fresneda I, Lloberas N, Riera M, Ma CJ, Ma GJ. Promising effects of ischemic preconditioning in renal transplantation. Kidney Int. 2002 Jun;61(6):2218–2227.
- Rasoulian B, Mohammadhosseniakbari H, Kadkhodaee M, Preconditioning with oxygen attenuates rat renal ischemia-reperfusion injury. J Surg Res. 2008 May 15;146(2):282–288.
- Cetin R, Devrim E, Kilicoglu B, Avci A, Candir O, Durak I. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: Possible protective roles of natural antioxidant foods. J Appl Toxicol. 2006 Jan;26(1):42–46.
- Tahepold P, Valen G, Starkopf J, Kairane C, Zilmer M, Vaage J. Pretreating rats with hyperoxia attenuates ischemia-reperfusion injury of the heart. Life Sci. 2001 Feb 23;68(14): 1629–1640.
- Tinits P. Oxygen therapy and oxygen toxicity. Ann Emerg Med. 1983 May;12(5):321–328.
- Shimeda Y, Hirotani Y, Akimoto Y, Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biological and Pharmaceutical Bulletin. 2005 Sep;28(9):1635–1638.
- Behling EB, Sendao MC, Francescato HD, Antunes LM, Costa RS, Bianchi Mde L. Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep. 2006 Jul–Aug;58(4):526–532.
- Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970 Mar;34:30–38.
- Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209–220.
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978 Nov 15;90(1):37–43.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7; 72:248–254.
- Solez K, Kramer EC, Fox JA, Heptinstall RH. Medullary plasma flow and intravascular leukocyte accumulation in acute renal failure. Kidney Int. 1974 Jul;6(1):24–37.
- Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998 Jun;131(6):518–526.
- Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: A review of some recent research. Food Chem Toxicol. 2006 Aug;44(8):1173–1183.
- Appenroth D, Winnefeld K, Schroter H, Rost M. Beneficial effect of acetylcysteine on cisplatin nephrotoxicity in rats. J Appl Toxicol. 1993;13(3):189–192.
- Sheikh-Hamad D, Timmins K, Jalali Z. Cisplatin-induced renal toxicity: Possible reversal by N-acetylcysteine. J Am Soc Nephrol. 1997 Oct;8(10):1640–1645.
- Nisar S, Feinfeld DA. N-acetylcysteine as salvage therapy for cisplatin nephrotoxicity. Ren Fail. 2002 Jul; 24(4):529–533.
- Ajith TA, Jose N, Janardhanan KK. Amelioration of cisplatin induced nephrotoxicity in mice by ethyl acetate extract of a polypore fungus, Phellinus rimosus. J Exp Clin Cancer Res. 2002 Jun;21(2):213–217.
- Bergendi L, Benes L, Durackova Z, Ferencik M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999; 65(18–19):1865–1874.
- Bougle D, Vert P, Reichart E, Hartemann D, Heng E. Retinal superoxide dismutase activity in newborn kittens exposed to normobaric hyperoxia effect of vitamin E. Pediatric Research. 1982;16(5):400.
- Bigdeli MR, Hajizadeh S, Froozandeh M, Normobaric hyperoxia induces ischemic tolerance and upregulation of glutamate transporters in the rat brain and serum TNF-alpha level. Exp Neurol. 2008 Aug;212(2):298–306.
- Esmaili Dehaj M, Baharvand B, Rasoulian B, Delayed protective effects of hyperoxia against cardiac arrhythmias and infarction in anesthetized rats. J Surg Res. 2009 Jan; 151(1):55–61.
- Dong H, Xiong L, Zhu Z, Chen S, Hou L, Sakabe T. Preconditioning with hyperbaric oxygen and hyperoxia induces tolerance against spinal cord ischemia in rabbits. Anesthesiology. 2002 Apr;96(4):907–912.
- Atasoyu EM, Yildiz S, Bilgi O, Investigation of the role of hyperbaric oxygen therapy in cisplatin-induced nephrotoxicity in rats. Arch Toxicol. 2005 May;79(5):289–293.
- Greeger R, Windhorst U. Comprehensive human physiology. 1st ed. Berlin, Heidelberg: Springer-Verlag; 1996.
- Aydinoz S, Uzun G, Cermik H, Effects of different doses of hyperbaric oxygen on cisplatin-induced nephrotoxicity. Ren Fail. 2007;29(3):257–263.
- Vera G, Chiarlone A, Cabezos PA, Pascual D, Martin MI, Abalo R. WIN 55,212-2 prevents mechanical allodynia but not alterations in feeding behaviour induced by chronic cisplatin in the rat. Life Sci. 2007 Jul 19;81(6): 468–479.
- Kim SH, Hong KO, Hwang JK, Park K-K. Xanthorrhizol has a potential to attenuate the high dose cisplatin-induced nephrotoxicity in mice. Food and Chemical Toxicology. 2005 Jan;43(1):117–122.
- Yu YN, Chen H, Li Y. Protect effect of bicyclol on cisplatin-induced nephrotoxicity in mice. Arch Toxicol. 2009 Apr; 83(4):381–387.
- Tüközkan N, Erdamar H, Seven I, Anabilim GT, Dal A. Measurement of total malondialdehyde in plasma and tissues by high-performance liquid chromatography and thiobarbituric acid assay. Firat Tip Dergisi. 2006;11: 88–92.
- Gaedeke J, Fels LM, Bokemeyer C, Mengs U, Stolte H, Lentzen H. Cisplatin nephrotoxicity and protection by silibinin. Nephrol Dial Transplant. 1996 Jan;11(1):55–62.
- Teicher BA, Lazo JS, Sartorelli AC. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981 Jan;41(1):73–81.
- Alagoz T, Buller RE, Anderson B, Evaluation of hyperbaric oxygen as a chemosensitizer in the treatment of epithelial ovarian cancer in xenografts in mice. Cancer. 1995 May 1;75(9):2313–2322.
- Cannizzaro A, Verga Falzacappa CV, Martinelli M, Misiti S, Brunetti E, Bucci B. O(2/3) exposure inhibits cell progression affecting cyclin B1/cdk1 activity in SK-N-SH while induces apoptosis in SK-N-DZ neuroblastoma cells. J Cell Physiol. 2007 Oct;213(1):115–125.
- Stogner SW, Payne DK. Oxygen toxicity. Ann Pharmacother. 1992 Dec;26(12):1554–1562.