Abstract
Chronic kidney disease is a worldwide health problem. Type II diabetes mellitus is now a major cause of end stage renal disease. The effect of diabetes mellitus through the dysregulation of the innate immunity results in increased tumor necrosis factor-α. This can lead to an increasing protein trafficking through the glomerular capillary, which can have an intrinsic renal toxicity. Seventy-four patients with type II diabetes mellitus with overt proteinuria were included in the study. They were randomly assigned to two groups of 37 patients (group 1: captopril 25 mg three times a day, group 2: captopril 25 mg and pentoxifylline 400 mg each three times per day). In the course of the study, two patients were excluded from each group. Daily urinary protein excretion was assessed at baseline and at two and six months. The reduction of urinary protein to creatinine clearance ratio in group 2 was 15.16 points more than in group 1 from baseline to the end of the study (p = 0.001). The difference in reduction only started after two months of pentoxifylline use. The differences in HbA1c and duration of diabetes mellitus at baseline in the two groups had not adversely affected the outcome of the study. There was a modest decrease in systolic blood pressure in group 2 as well (p = 0.041). Combining an angiotensin-converting enzyme inhibitor and pentoxifylline can lead to a greater reduction in proteinuria.
INTRODUCTION
Chronic kidney disease is a worldwide health problem. With 850,000 deaths per year and more than 15,000,000 disability-adjusted life years, diseases of the kidney and the urinary tract have a significant burden on the population.Citation[1] Type II diabetes mellitus is now a major cause of end stage renal disease in the world.Citation[2] Diabetes mellitus (DM) is a metabolic disorder that leads to a number of complications. The etiology of these complications is multifactorial; however, dysregulation of the innate immunity leading to an increased inflammatory response seems to be a common feature. This response raises the level of a number of cytokines such as tumor necrosis factor alpha (TNF-α).Citation[3]
Proteinuria is the single most powerful predictor of end stage renal disease (ESRD) in patients with type II DM and nephropathy.Citation[4] Protein overload per se activates proximal tubular epithelial cells in vitro culture to up-regulate genes encoding for endothelin, chemokines and cytokines.Citation[5] In an animal model, the renal expression of the main pro-inflammatory cytokine TNF-α in diabetic nephropathy is significantly correlated with urinary albumin excretion.Citation[6] Relatively independent of the initial type of insult, chronic proteinuric nephropathies ultimately lead to ESRD.Citation[7] The amount of urinary proteins correlates with disease progression more than the underlying pathology, which is due to the toxic effect of protein passage across the glomerular membranes.Citation[8] More albuminuria causes greater renal damage, and a reduction of albuminuria by therapy would have a renoprotective effect. The importance of this is demonstrated in that the residual albumin excretion at six months after therapy is as predictive of outcome as is baseline excretion.Citation[9] In this study, we evaluated a new combination of captopril and pentoxifylline to decrease protein excretion in patients with type II diabetes mellitus with overt proteinuria.
METHODS
Study Subjects
All patients with type II DM and overt proteinuria (>500 mg in a 24-h urine sample collection) who had regular follow-ups at an endocrinology and nephrology clinic were evaluated for inclusion into the study.
Inclusion criteria for the study included absence of kidney or urinary tract disease other than proteinuria and controlled blood pressure (≤140/90) with medication other than ACEI, ARBs, and/or non-dihydropyridine calcium channel blockers, as determined from blood pressure recording from the last three visits in the past six months. Blood pressure was measured two times in each visit with a five-minute interval after five minutes of rest in the sitting position. A well-controlled blood sugar level (HbA1c ≤ 7.5%), adhering to the diet protocol for patients with renal disease, was provided by a certified nutritionist in the clinic. Adherence to the diet was controlled by the nutritionist. Patients were excluded if they had New York Heart Association functional class III or IV, valvular heart disease via auscultation tone, unstable angina, myocardial infarction, cerebrovascular accidents, psychiatric disease, and prior allograft kidney transplant according to the patient's history or routine physical exam in office visits. Further exclusion criteria included acute illness; infectious disease including urinary tract infection, leukocytosis, or any febrile illness at enrollment; prior history or development of any form of malignancy; history of alcohol or drug abuse or smoking; pregnancy; allergy to derivatives of methyl xanthines; or current pentoxyphilline use. Furthermore, it was decided that patients undergoing surgery during the study would be excluded.
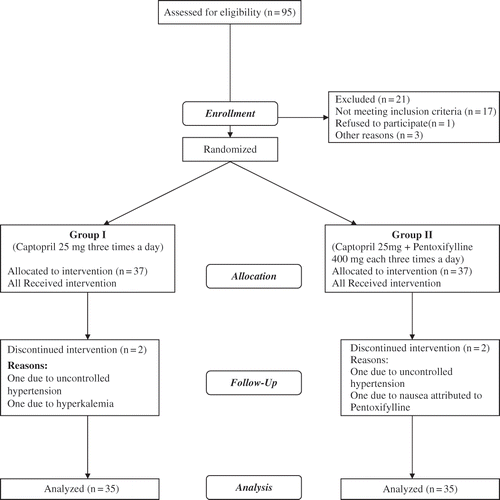
Ninety-five patients were evaluated for participation in the trial. After obtaining consent and reassurance that the participants were willing to follow the protocol of the study, a series of tests was requested for the 74 remaining participants regarding HbA1c (HPLC method), fasting blood glucose, complete blood count, TG, LDL-c, 24 h urine collection (for volume, creatinine, and protein according to the turbimetric method), serum BUN, creatinine, sodium, potassium, urine analysis, and culture.
After receiving lab results and demographic data, the 74 patients were randomly allocated into two groups of 37 in an open label fashion (see ). Group 1 received captopril 25 mg (Iran Daru Company, Tehran, Iran; IRC: 1228078583) three times daily, and group 2 received a combination of captopril 25 mg three times daily and PTF 400 mg (Extended Release, Hakim Pharmaceutical Co., Tehran, Iran; IRC: 1228080449) three times daily.
Each medication regime was given randomly to patients by the clinic's secretary so that clinicians and patients were unaware of their grouping. The medications were paid for by the grant provided to the project and were not complimentary of the pharmaceutical manufacturing companies. In follow-up after two and six months, labs were repeated. During the study, four participants were excluded: one person from each group was excluded due to uncontrolled hypertension, one from group 1 due to hyperkalemia as a result of captopril use, and one in group 2 due to nausea and vomiting as a result of PTF use. As a result, 35 participants were available in each group until the end of the study.
Statistical Analysis
The data was entered into the SPSS software (evaluation version 15 for Windows, Chicago, Illinois, USA). Outcome was compared using the standard ANOVA variance and Bonferoni/Dunn post hoc correction. For comparing means and percentages, the student's and/or paired t-tests and chi-squared test were used. To assess the correlation of variables with changes in 24 h urinary protein excretion, multiple regression analysis was used. Data are provided as mean ± SD. p value was deemed significant below 0.05.
RESULTS
Initial demographic data of the two groups showed them to be without any significant differences (see ). Variables at the end of the study are provided in .
Table 1 Patient demographics and initial laboratory and clinical evaluations
Table 2 Patient laboratory and clinical evaluations at the end of the study
Of all variables at baseline, the two groups differed in duration of DM and HbA1c, which may have a compromising effect on the study due to the effect of metabolic control and renal protein excretion. However, both groups had their HbA1c levels in an acceptable level, and further analysis showed that changes in HbA1c was similar in the two groups from baseline to the end of the study (p = 0.497, see ), and in multiple regression analysis no significant correlation was found between HbA1c levels and changes in urinary protein excretion (p = 0.77). It seems unlikely that the difference observed in HbA1c in the beginning until the end of the study in the two groups could be attributed to better glycemic control due to the short-term nature of the follow up.Citation[10]
Table 3 Differences observed in HbA1C and urinary protein excretion during the study
At the end of the study, urinary protein excretion was significantly lower in group 2. Systolic blood pressure (SBP) was lower in group 2 as well; however, multiple regression did not show a significant correlation between systolic blood pressure and changes in urinary protein excretion (p = 0.903).
Of note was that urinary protein excretion did increase in four cases in group 1, and a less than 15% decrease was observed in four others. In group 2, this increase was seen only in one case, and two cases showed a decrease of less than 15%. Laboratory values regarding HbA1C and urinary protein have been compared in and . The change in HbA1C from the beginning to the end of the study was not significantly different between the two groups, and it can be expected that the difference in glycemic control did not cause the observed difference in urinary protein excretion.
Table 4 Comparing the amount of urinary protein excretion at the beginning and at the end of the study
The reduction in urinary protein excretion was similar in the two groups until the end of the second month, while at six months, the reduction in group 2 (pentoxifylline + captopril) is nearly three-fold that of group 1 (captopril).
In multiple regression analysis, age, sex, length of DM, serum creatinine, triglycerides (TG), cholesterol, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were not significantly associated with the change in urinary protein excretion, while a strong association was observed with the type of medical therapy (p = 0.006).
DISCUSSION
It was Hasegawa et al. who first proposed a role for the pro-inflammatory cytokines TNF-α and interleukin-1 contributing to the pathogenesis of diabetic nephropathy.Citation[11] This cytokine mediates inflammatory reactions and hemodynamic changes leading to a decreased GFR and albuminuria secondary to damage of the glomerular permeability barrier. TNF-α has a pathogenic role in the development of renal injury in diverse renal disease including diabetic nephropathy, making it a potential therapeutic target.Citation[12]
Pentoxifylline (PTF) is a methylxanthine phosphodiesterase inhibitor with significant hemorheological effects. It reduces blood viscosity via increased leukocyte and erythrocyte deformability and decreased neutrophil adhesion/activation, and it improves peripheral tissue oxygenation presumably through enhanced blood flow. It is used clinically to treat patients with intermittent claudication on the basis of chronic occlusive arterial disease of the limbs. In addition to its hemorheological properties, PTF can significantly reduce renal TNF-α expression, synthesis, and excretion during experimental DM, causing a decrease in urinary albumin excretion.Citation[12] Pentoxifylline reduces the excretion of both high and low molecular-weight urinary proteins.Citation[13] A significant improvement of the hemorheologic pattern and a significant marked reduction of albumin excretion rate and proteinuria have been found in diabetic patients treated with PTF, independently of the degree of metabolic control.Citation[14] The dosage used in this trial was the same as a previously published article,Citation[15] which is almost three times the recommended dose for patients with renal impairment considering the accumulation of PTF and its metabolites.Citation[16] Despite this, we experienced almost no side effects except one case in group 2, whose nausea and vomiting was attributed to PTF usage.
There is a clear renoprotective benefit to using angiotensin receptor blockers in type II diabetic patients.Citation[17] There is also good evidence to support treatment with angiotensin-converting enzyme inhibitors (ACEI) to reduce the progression of nephropathy in patients with type II DM.Citation[18] The benefits of ACEI or angiotensin receptor blockers (ARBs) on renal outcomes in placebo-controlled trials probably result from a blood-pressure-lowering effect. In patients with DM, additional renoprotective actions of these substances beyond lowering blood pressure remain unproven.Citation[19] In terms of renal protection, there are ample data to support a role for both ACEI and ARB to prevent the progression from microalbuminuria to overt albuminuria in both type I and type II DM. However, when progression of renal disease is used as an end point, protection had earlier been demonstrated with ACEI only for type 1 but not type II DM. In this latter group, only ARB has been shown to slow progression to ESRD.Citation[20] More recently, ACEIs have shown similar effects when compared to ARBs in the DETAIL trial.Citation[21] Angiotensin receptor blockers have failed to show a significant reduction in total mortality and cardiovascular morbidity and mortality in diabetic patients. The only statistical benefit was the reduction of end-stage renal disease compared with placebo. Therefore, at this time ARBs have not proved to be superior to standard anti-hypertensive treatment in diabetic patients.Citation[22]
In this study, we used a combination of an ACEI and PTF. Previously, this combination had only been used once.Citation[23] In a trial comparing the individual effects of these drugs, both had significantly reduced proteinuria.Citation[24] Pentoxifylline was evaluated in a randomized controlled trial of 61 patients with significant residual proteinuria after one year of therapy with an ARB.Citation[14] At four months, the combination of PTF and an ARB led to a significant reduction in proteinuria (-16.7 percent) versus an increase with ARB alone (+5.5 percent). Randomized controlled trials comparing PTF to captopril and placebo have reported similarly favorable findings.Citation[13,Citation23–25] An early study in 1987 conducted by Solerte et al. on a group of insulin-dependent patients with overt diabetic nephropathy showed that in one year there was a marked reduction of albuminuria, proteinuria, SBP, and DBP, which ultimately led to an improvement of creatinine clearance.Citation[14] In contrast, in our study, at least in a six-month follow-up, only SBP and proteinuria decreased without creatinine clearance or DBP being affected significantly.
The power of PTF and ACEI is similar in reducing proteinuria with a significant impact for PTF only in overt proteinuria (protein > 300 mg/d).Citation[26] The findings of our study showed that PTF has an additive effect when compared to captopril alone in lowering proteinuria. The effect starts after two months of administering the drug and is evident by six months. The fact that the effect of PTF starts no sooner than two months had not been documented previously to our knowledge. After six months, PTF reduced daily protein excretion by 55% in group 2 and only 27% in group 1 (p = 0.001; see ), the former being in accordance with the current practice trends of 60% reduction from baseline. A urinary protein-to-creatinine clearance ratio was created to adjust the protein excretion, which showed a 15.16 excess points of ratio in group 2 (p < 0.001; see ). Despite both groups being below the target blood pressure of 130/80, SBP was decreased in both groups but further reduced by PTF and captopril combined when compared to captopril alone (p = 0.041). Changes in DBP were not significant in six months. In one study, with PTF used alone, a reduction in DBP was seen in a one-year follow-upCitation[14]; however, it is SBP and not DBP that has been shown to decrease complications. Each 10 mmHg reduction in SBP is associated with a 12 percent risk reduction in diabetic complications (p < 0.001). The lowest risk occurs at a SBP below 120 mmHgCitation[27]; however, further reductions of SBP and DBP beyond 120 and 85 respectively may potentially have an adverse effect.Citation[28] Although PTF alone does not decrease SBP or DBP,Citation[26] the group receiving PTF combined with ACEI had a modest difference in lowering SBP more at the end of six months when compared to the group treated only with an ACEI (p = 0.041).
Analysis from the RENAAL study showed that SBP or albuminuria reduction was associated with a lower risk for ESRD; when both SBP and albuminuria are reduced, it is associated with the lowest risk for events. Therefore a dual strategy, targeting both SBP and albuminuria reduction, should be considered for maximum impact in antihypertensive treatment to improve renal outcomes in patients with diabetic nephropathy.Citation[29]
PTF by itself does not have an impact on SBP, DBP, or glomerular filtration rateCitation[26]; therefore, an antihypertensive agent will be required. Drugs with the ability to block the renin-angiotensin system can give two benefits: lowering blood pressure while lowering daily protein excretion. This makes PTF combined with an ACEI or an ARB a good candidate, as it has a considerable impact without any major side effects even at high doses.
In conclusion, we believe that a reduction in SBP and daily protein excretion should both be targeted in patients with diabetic nephropathy. We recommend the combination of ACEI and PTF, as it achieves both targets with minimal side effects. ARBs can also be used as a replacement of ACEI in this setting.
ACKNOWLEDGMENTS
The authors acknowledge thesis grant number 85-3077 from Shiraz University of Medical Sciences (thesis registration number 2433) and the Shiraz Nephrology Urology Research Center, Shiraz University of Medical Sciences, Shiraz, Fars, Iran.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- World Health Organization Burden of disease project. Available at http://www3.who.int/topics/global_burden_of_disease/en/ (December 2008).
- Atkins RC. The changing patterns of chronic kidney disease: The need to develop strategies for prevention relevant to different regions and countries. Kidney Int Suppl. 2005;68 (Suppl. 98):S83–S85.
- Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227–1239.
- Keane WF, Lyle PA. Reduction of endpoints in NIDDM with the Angiotensin II Receptor Antagonist Losartan study. Recent advances in management of type 2 diabetes and nephropathy: Lessons from the RENAAL study. Am J Kidney Dis. 2003;41 (Suppl. 1):S22–S25.
- Zoja C, Benigni A, Remuzzi G. Protein overload activates proximal tubular cells to release vasoactive and inflammatory mediators. Exp Nephrol. 1999;7:420–428.
- Navarro JF, Milena FJ, Mora C, Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562–570.
- Ruggenenti P, Remuzzi G. The role of protein traffic in the progression of renal diseases. Annu Rev Med. 2000;51:315–327.
- Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15.
- de Zeeuw D, Remuzzi G, Parving HH, Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int. 2004;65:2309–2320.
- Bilous RW, Mauer SM, Sutherland DE, The effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med. 1989;321:80–85.
- Hasegawa G, Nakano K, Sawada M, Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40:1007–1012.
- Navarro JF, Mora-Fernández C. The role of TNF-alpha in diabetic nephropathy: Pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17:441–450.
- Rodriguez-Morán M, González-González G, Bermúdez-Barba MV, Effects of pentoxifylline on the urinary protein excretion profile of type 2 diabetic patients with microproteinuria: A double-blind, placebo-controlled randomized trial. Clin Nephrol. 2006;66:3–10.
- Solerte SB, Fioravanti M, Patti AL, Pentoxifylline, total urinary protein excretion rate and arterial blood pressure in long-term insulin-dependent diabetic patients with overt nephropathy. Acta Diabetol Lat. 1987;24:229–239.
- Navarro JF, Mora C, Muros M, García J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: A short-term, randomized, controlled trial. J Am Soc Nephrol. 2005;16: 2119–2126.
- Paap CM, Simpson KS, Horton MW, Schaefer KL, Lassman HB, Sack MR. Multiple-dose pharmacokinetics of pentoxifylline and its metabolites during renal insufficiency. Ann Pharmacother. 1996;30:724–729.
- Hostetter TH. Prevention of end-stage renal disease due to type 2 diabetes. N Engl J Med. 2001;345:910–912.
- Hamilton RA, Kane MP, Demers J. Angiotensin-converting enzyme inhibitors and type 2 diabetic nephropathy: A meta-analysis. Pharmacotherapy. 2003;23:909–915.
- Berl T. Angiotensin-converting enzyme inhibitors versus AT1 receptor antagonist in cardiovascular and renal protection: The case for AT1 receptor antagonist. J Am Soc Nephrol. 2004; 15(Suppl. 1):S71–S76.
- Casas JP, Chua W, Loukogeorgakis S, Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: Systematic review and meta-analysis. Lancet. 2005;366:2026–2033.
- Barnett AH, Bain SC, Bouter P, Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351: 1952–1961.
- Siebenhofer A, Plank J, Horvath K, Angiotensin receptor blockers as anti-hypertensive treatment for patients with diabetes mellitus: Meta-analysis of controlled double-blind randomized trials. Diabet Med. 2004;21:18–25.
- Adler AI, Stratton IM, Neil HA, Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ. 2000;321:412–419.
- Aminorroaya A, Janghorbani M, Rezvanian H, Aminian T, Gharavi M, Amini M. Comparison of the effect of pentoxifylline and captopril on proteinuria in patients with type 2 diabetes mellitus. Nephron Clin Pract. 2005;99:73–77.
- Rodríguez-Morán M, Guerrero-Romero F. Pentoxifylline is as effective as captopril in the reduction of microalbuminuria in non-hypertensive type 2 diabetic patients—a randomized, equivalent trial. Clin Nephrol. 2005;64:91–97.
- McCormick BB, Sydor A, Akbari A, Fergusson D, Doucette S, Knoll G. The effect of pentoxifylline on proteinuria in diabetic kidney disease: A meta-analysis. Am J Kidney Dis. 2008;52:454–463.
- Harmankaya O, Seber S, Yilmaz M. Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren Fail. 2003;25:465–470.
- Berl T, Hunsicker LG, Lewis JB, Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16: 2170–2179.
- Eijkelkamp WB, Zhang Z, Remuzzi G, Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: Post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18:1540–1546.