Abstract
Background: The aim of this study was to investigate the correlation between the transforming growth factor (TGF)-β1 gene −509C/T polymorphism and the susceptibility to primary nephrotic syndrome (PNS), and in particular to the severe degree of tubulointerstitial damage (TID) seen in Chinese. Methods: Ninety-eight PNS patients and 128 normal controls were studied. The extent of tubulointerstitial changes was evaluated and patients were divided into two groups according to the severe or mild degree of TID. The TGF-β1gene −509C/T polymorphism was detected with polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique, and the serum level of TGF-β1 was determined by enzyme-linked immunosorbent assay (ELISA). Results: No statistical differences in genotype or allele frequency of the TGF-β1 gene −509C/T were found between PNS and normal subjects. However, T allele and CT + T T genotype frequency were higher in the PNS with severe TID than the mild TID and controls. Additionally, the serum concentration of TGF-β1 was significantly higher in the PNS with severe TID group than the other two groups and in the T T genotype individuals than the CC and CT genotype individuals. A logistic regression analysis demonstrated that TGF-β1 gene −509C/T genotype was the risk factor of TID in PNS patients [OR (odd ratio) 2.34, confidence interval (CI) 0.98–3.46, p = 0.012]. Conclusion. TGF-β1 gene −509C/T polymorphism was associated with severe TID. The higher value in serum concentration of TGF-β1 was also associated with severe TID and the T T genotype/T allele. T allele gene might be the important risk factor for susceptibility.
INTRODUCTION
Tubulointerstitial damage (TID) is not only a concomitant symptom of glomerular disease but also can lead directly to renal function decline independent of glomerular lesion. It has been reported that urinary protein can participate in the pathological process of TID as an independent etiological factor and can accelerate the progression of end-stage renal disease (ESRD).Citation1,Citation2 Our previous study reported that the excretion of urinary IgG and the proteinuria selectivity index based on IgG were significantly associated with the severity of TID in adult-onset primary nephrotic syndrome (PNS) in China.Citation3 These clinical parameters might be useful as independent risk factors to predict the development and progression of proteinuric nephropathy. Numerous studies have also demonstrated that transforming growth factor (TGF)-β1 is the main cytokine leading to glomerulosclerosis and interstitial fibrosisCitation4 and that the expression of TGF-β1 in kidney tissue can be promoted by urinary protein filtration across the glomeruli.Citation5 However, TGF-β1 gene polymorphism contributes to the transcription level of TGF-β1 mRNA, as well as to the activation and serum secretion of TGF-β1 protein. Such polymorphism might therefore be useful as markers of progression of renal function failure.Citation6,Citation7 Khalil et al.Citation8 reported a significant association between TGF-β1 gene polymorphism and the progression of chronic kidney failure and he demonstrated that TGF-β1 single nucleotide polymorphism (SNP) may be useful as prognostic indicators for the progression of chronic kidney failure. Until this study, however, there were no reports of whether the TGF-β1 gene −509C/T polymorphism correlates with PNS characterized by a great deal of urinary protein and the severe degree of TID, nor were there reports of this polymorphism's correlation with the serum level of TGF-β1 in PNS patients. This study utilizes the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique to compare the TGF-β1 gene −509C/T mononucleotide polymorphism of PNS patients with that of normal persons and to explore the relationship of TGF-β1 gene polymorphism and the incidence of PNS and severe TID.
METHODS
Subjects
Ninety-eight [53 (54%) male, 45 (46%) female] patients with a diagnosis of PNS were selected between April 2005 and December 2006. All subjects were members of the Han population of China's Hunan province and were selected from the Division of nephrology, second Xiangya hospital, Central South University. Average age at the time of biopsy was 29.69 ± 13.10 years. Nephrotic syndrome (NS) was defined as proteinuria ≥3.5 g/day and serum albumin ≤3.0 g/dL, with or without edema and hyperlipidemia. Possible secondary causes such as infection, autoimmune disease, metabolic disease, or toxic disease were excluded in all cases. The estimated glomerular filtration rate (eGFR) according to modification of diet in renal disease (MDRD) formula of the patients chosen in this study were all more than 90 mL/min/1.73 m2. All the subjects in our study had taken renal biopsies and were diagnosed as PNS. The interval between the apparent onset of the PNS and the time of renal biopsy in all patients ranged from 5 days to 3 months. None of the patients had complications of acute renal failure, thrombosis, or severe infection. All the subjects did not take the medicine that can influence serum TGF-β1 levels such as angiotension converting enzyme inhibitors (ACEI), and so on. One hundred and twenty-eight cases were chosen as normal controls, including 73 males and 58 females. Their average age was 27.45 ± 9.87 years. This study was approved by the patients and the Ethics Committee of Central South University.
Renal pathological evaluation
Paraffin sections for light microscopy were stained by the hematoxylin–eosin, periodic acid-Schiff reaction, Masson's trichrome, and periodic acid silver methylamine methods. Histopathological evaluation was carried out by a single investigator who was not aware of the disease status of the subjects from whom the samples originated. A semiquantitative evaluation of histological parameters was performed at the same time patients underwent renal biopsy. The extent of tubulointerstitial changes was evaluated according to previously published methods.Citation3 Ten consecutive microscopic fields of the cortex of each biopsy sample were examined with an objective of magnification ×40. A field was graded 0 if there was no tubular atrophy, 1 if tubular atrophy was focal, and 2 if tubular atrophy was diffuse. Similarly, interstitial fibrosis and/or infiltration were graded 0, 1, or 2 if absent, focal, or diffuse, respectively. Tubular and interstitial scores were added to obtain a single score for TID, which was then classified as follows: 0 or 1, lesions absent or very mildly focal; 2, focal tubular and interstitial lesions; and 3 or 4, diffuse lesions. Patients were divided into two groups such as severe TID and mild TID groups according to the degree of TID. Patients with a score of less than 2 (n = 62) in mild TID group were compared to patients with a score of greater than or equal to 2 (n = 36) in severe TID group.
Measurement of serum TGF-β1 concentration
In 98 patients with PNS and 128 normal controls whose serum samples at the time of diagnosis were available, serum levels of TGF-β1 were measured by an enzyme-linked immunosorbent assay (ELISA) kit (Jin Mei Biotechnological Limited Company, Shenzhen, China). A monoclonal antibody specific for TGF-β1 was precoated onto a microplate. Standards and samples were pipetted into the wells and any TGF-β1 present was bound by the immobilized antibody. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells, resulting in color development in proportion to the amount of TGF-β1 bound in the initial step. The color development was stopped and the intensity of the color was measured by spectrophotometer at 450 nm. The results were calculated by reference to a standard curve and expressed as picograms per milliliter (pg/mL).
Screening for gene polymorphism
Genomic DNA of peripheral blood cells was isolated by using a standard phenol/chloroform isolation procedure. The genotypes of the −509 position of the TGF-β1 gene (C or T) were determined by PCR-RFLP as described previously.Citation9 Reaction mixtures of 25 μL were prepared containing 100 ng DNA, 2 U Taq polymerase, 0.1 mM dNTPs, 10× PCR buffer (10 mM Tris HCl, pH 8.3, 50 mM KCl), 1.0 mM MgCl2 (Bao Biological Limited Company, Dalian, China), and 40 pM of each primer (Ruijing Biotechnological, Shanghai, China). The forward primer sequence was GGGGACACCATCTACAGTG and the reverse primer sequence was GGAGGAGGGGGCAACAGG. PCR was performed with an initial denaturation at 94°C for 5 min. This was followed by 35 cycles, with denaturation at 94°C for 30 s, annealing at 60°C for 30 s, then elongation at 72°C for 30 s. A final elongation of 5 min at 72°C then followed. An amplification check was carried out using electrophoresis in a 2% agarose gel in 0.5% TAE buffer containing 0.25 mL ethidium bromide (Boya Bioengineering Limited Company, Shanghai, China) with a voltage of 80 V for 30 min. Eight microliters of amplification product and 1.6 μL of loading buffer were added to each well.
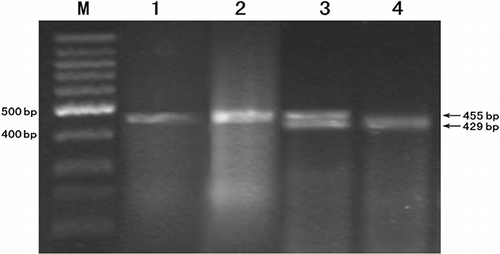
Ten microliters of the amplification product was digested with 4 U Eco81 I restriction endonuclease (Tianwei Time Company, Beijing, China). The reaction mixture was incubated at 37°C for 6 hours. PCR products digested by restriction enzyme were subjected to electrophoresis in a 2% agarose gel at 80 V for 30 min. Using ultraviolet trans-illumination after ethidium bromide staining, the products were visualized and their sizes were determined using a DNA ladder. A gel of the RFLP digestion products is shown in .
Statistical analysis
Data analyses were performed with the Statistical Package for the Social Sciences (SPSS, version 11.5). Data were expressed as mean ± SD. The significance level for statistical tests was set at p < 0.05. Hardy–Weinberg equilibrium analysis was assessed by χ2 test and used to test whether the balance of genotypes observed was consistent with that expected from a population in equilibrium. Differences between the patients with PNS and the controls with respect to the allele frequencies and genotype distributions were analyzed by χ2 test. The one-way analysis of variance (ANOVA) test was used to compare continuous data among patients with different genotypes. Statistical analyses between the two groups were performed by the two-tailed unpaired Student's t-test. ORs and 95% confidence interval (CI) for those per-genotype and per-allele analyses was assessed by logistic regression.
RESULTS
General condition of different groups
There were no obvious differences in age, gender, systolic blood pressure, and diastolic blood pressure between normal control and PNS patients and between the groups in severe TID and mild TID (p > 0.05). Except 24 h urine protein, blood urea nitrogen, serum creatinine, and eGFR in normal control were also similar to those in PNS patients (p > 0.05). Except eGFR, above parameters in the severe TID group was higher than those in the mild TID group, but only serum creatinine had statistical significance (p < 0.05) (see and ).
TABLE 1. Comparison on general condition in PNS patients and normal controls
TABLE 2. Comparison on general condition in mild TID and servere TID patients
−509C/T genotype analysis of the TGF-β1 gene
The length of the TGF-β1 gene product is 455 bp, with a −509C/T mononucleotide polymorphism on the amplification fragment. The CC genotype yields only one fragment of 455 bp. The TT genotype yields two fragments of 429 bp and 26 bp. The CT genotype yields three fragments with 455 bp, 429 bp, and 26 bp. The fragment with 26 bp cannot be seen on the gel if electrophoresis is run long enough to visualize the larger bands (see ).
Comparison of genotype and allele frequencies for the TGF-β1 gene in PNS patients
In the normal control group, C allele frequency was 57.4% and T allele frequency was 42.6%. In the PNS patient group, C allele frequency was 55.1% and T allele frequency was 44.9%. Applying the above data to the law of Hardy–Weinberg, expected values were obtained. There was no significant difference between the actual and expected frequencies in the two groups (χ2 = 1.0295, p = 0.598; χ2 = 0.550, p = 0.760). This indicated that the −509C/T TGF-β1 genotype frequency in the Han Chinese population is consistent with Hardy–Weinberg balance.
Our study also found no significant difference in the genotype and allele frequencies of TGF-β1 between the groups of PNS patients and normal controls (χ2 = 2.164, p = 0.266; χ2 = 1.213, p = 0.178) (see ).
TABLE 3. Compare of the distribution of the frequencies of TGF-β1 genotype and alleles in PNS and control (n, %)
Relationship between TGF-β1 genotype and degree of TID in PNS patients
There were significant differences in genotype and allele frequencies between the severe TID group and the mild TID group (χ2 = 1.119, p = 0.023; χ2 = 2.109, p = 0.034). The frequencies of TT genotype and T allele in the severe TID group were higher than those in the mild TID group (25% vs. 9.7%; 47.3% vs. 27.4%) (see ).
TABLE 4. Compare of the distribution of the frequencies of TGF-β1 genotype and alletes in the patients with mild TID and severe TID (n, %)
A logistic regression analysis on risk factors of TID in PNS patients
To test for associations with susceptibility to TID in PNS patients, related risk factors and genotype distributions were compared by logistic regression analysis. There were no significant association between age, gender, smoking, drinking, systolic pressure, diastolic pressure, proteinuria, and TID. However, serum creatinine and TGF-β1 −509C/T genotype were associated with TID (OR 3.14, CI 2.13–6.78, p = 0.004; OR 2.34, CI 0.98–3.46, p = 0.012) (see ).
TABLE 5. A logistic regression analysis on risk factors of TID in PNS patients
Comparison of TGF-β1 serum concentration in different groups
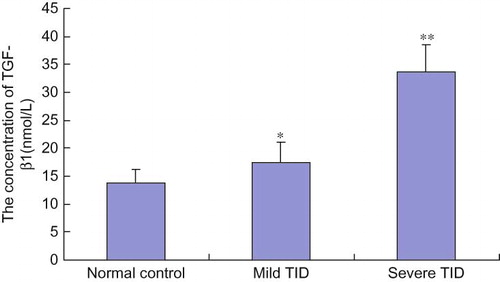
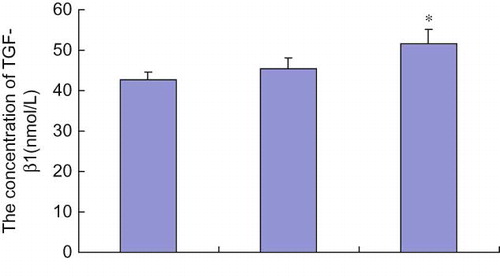
The TGF-β1 serum concentration was higher in the mild TID group than in the normal control group (p = 0.03), and TGF-β1 serum concentration in the severe TID group was higher than that in the mild TID group and the normal control group (p < 0.05) (see ). Correlation analysis demonstrated a clear association between TGF-β1 serum concentration and degree of TID, with the higher serum concentrations of TGF-β1 corresponding to more severe TID (r = 0.953). TGF-β1 serum concentration was also associated with TGF-β1 gene C/T polymorphism. TGF-β1 serum concentration in patients with TT genotype was higher than in patients with CT and CC genotypes. However, this difference was only statistically significant in the comparison of TT with CC genotypes (p = 0.02) (see ).
DISCUSSION
PNS is one of the most frequent syndromes characterized by heavy proteinuria. Many reported studies suggested a genetic susceptibility to PNS and reviewed many potential genetic factors. There was study on the role of the renin–angiotensin system, cytokines, and their respective receptors on the severity and clinical course of the disease in 2000, supporting to the immunogenetic basis of this disease.Citation10 Moreover, several authors have reported the presence of familial clustering, human leukocyte antigen (HLA) associations suggesting a genetic susceptibility to this disease.Citation11,Citation12 Recently, it was reported that majority of PNS occurs as a sporadic form, the incidence of familial cases is from 3% to 5%, and seven genes have been recognized till present, which mutations are responsible for severe forms of NS: NPHS1, NPHS2, ACTN4, CD2AP and WT1, TRPC6, and LAMB2.Citation13,Citation14 However, genetic susceptibility of TID in PNS has not been studied, and related genes involving in TID is still unclear.
Protein filtered through the glomerular capillary can be used as a marker of the severity of glomerular lesions. In addition, it may participate in the progression of TID as an independent etiological factor.Citation3,Citation15 TID is the pathological process common to all chronic renal diseases developing to ESRD, and the prognosis of kidney disease is determined by the degree of TID.Citation16 Therefore, searching for clinical factors associated with TID induced by proteinuria is of great significance for the determination of the development and prognosis of kidney disease. In a previous study, we found that excretion of urinary IgG and the proteinuria selectivity index based on IgG were significantly associated with the severity of TID in adult-onset PNS in China. These clinical parameters might be useful for the prediction of the development and progression of proteinuric nephropathy as independent risk factors. As useful noninvasive and easily repeatable indicators, they may assist in evaluating the outcome and prognosis of patients with PNS.Citation3 It has been reported that proteinuria can stimulate the renal tubule to secrete numerous inflammatory and growth factors. Among these, TGF-β1 is the main profibrotic factor, playing an important role in the interstitial fibrosis induced by proteinuria.Citation17,Citation18
The 30 KD TGF-β1 gene is situated on the long arm of the 19th chromosome and includes seven exons and six introns. TGF-β1 can increase the synthesis of extracellular matrix and inhibit its degradation. Moreover, TGF-β1 can promote the deposition of extracellular matrix through the upregulation of integrin expression on the cell surface. TGF-β1 can also stimulate tubular cells to generate chemotactic factors, such as MCP-1 and RANTES, leading to the activation of inflammatory reactions in the tubulointerstitium and the promotion of interstitial fibrosis. In addition, TGF-β1 can induce the transdifferentiation of tubular epithelial cells into fibroblasts. At least three SNPs have been identified in the TGF-β1 gene control region: −509 C/ T, −800 G/A, and −988 C/A. The TGF-β1 gene −509C/T SNP is located about 509 bp before the transcription initiation site, between −731 and −453, which has been identified as the negative control region of the TGF-β1 gene.Citation4 Despite many attempts to elucidate the relationship between the TGF-β1 gene −509C/T polymorphism and the susceptibility to nephropathy, a unified conclusion has not been reached. It has been reported that the TGF-β1 −509T allele is closely associated with the level of expression of TGF-β1 protein in tubular epithelial cells, the severity of proteinuria, and the infiltration of inflammatory cells in the renal interstitium. These associations indicate that it could be used to predict the progression of chronic renal failure.Citation5 Furthermore, some investigators found that the CT genotype of the TGF-β1 gene −509C/T was positively correlated with reflux nephropathy.Citation19 In a longitudinal study of the correlation between the TGF-β1 gene −509C/T polymorphism and IgA nephropathy, Lim et al.Citation20 observed 108 cases for more than 3 years and compared them with 55 healthy adults. The survival curve demonstrated a lower survival rate in TT genotype individuals than in CT + CC genotype individuals. Nevertheless, several other reports contradict with these findings. Cho et al.,Citation21 in an observation of 207 cases in Korea, found that the TGF-β1 gene −509C/T was unrelated to the incidence of chronic allograft nephropathy. Similarly, Carturan et al.,Citation22 in a study of 101 IgA nephropathy patients and 118 healthy adults, found no association between the TGF-β1gene −509C/T and IgA nephropathy. Sato et al.Citation23 compared the TGF-β1 gene −509C/T genotypes of 329 IgA nephropathy patients with 279 healthy adults and also found no relation between the genotype and the susceptibility to IgA nephropathy, although the CC genotype was correlated with proteinuria and proliferation of mesangial cells. Whether the TGF-β1 gene polymorphism and serum concentration of TGF-β1 are correlated with the extent of TID and morbidity of PNS, therefore, still remain unknown.
Our study found no difference between PNS patients and normal controls in the genotype and allele frequencies of the TGF-β1 gene −509C/T. However, when comparing the degree of TID and the genotype of the TGF-β1 gene −509C/T SNP, a significant disparity in genotype frequency existed between the mild TID and severe TID groups. The patients in the mild TID group were mainly CC genotype; the severe TID group exhibited a higher frequency of TT + CT genotypes. Moreover, we demonstrated that the investigated TGF-β1 gene −509C/T gene polymorphisms were significantly associated with the degree of TID in PNS patients by logistic regression analysis. In addition, we found that serum concentration of TGF-β1 was significantly higher in the severe TID group than in the other two groups. The level of serum TGF-β1 also differed according to the different genotypes of TGF-β1 gene −509C/T, with a significantly lower concentration in CC genotype patients than in CT and TT genotypes. The above data suggested that there was no correlation between genetic susceptibility to PNS and TGF-β1 gene −509C/T polymorphism, but that the polymorphism could provide references for predicting the extent of TID. The T alleles of the TGF-β1 gene promoter −509C/T locus might be a risk factor in PNS patients with TID, because TT genotype patients presented with a more severe degree of TID. Furthermore, TGF-β1 gene −509C/T polymorphism was related to the serum level of TGF-β1, illustrating that different TGF-β1 −509C/T genotypes can affect the expression of TGF-β1 protein. On the whole, we found that high serum level of TGF-β1 were correlated with TT + CT genotypes of TGF-β1 −509C/T, and TT + CT genotype or T allele were associated with severe TID. We hypothesized that TT + CT genotypes of TGF-β1 gene −509C/T promoted the production of TGF-β1 protein not only in the circulation but also in the kidney tissue, then TGF-β1 would trigger the increase of fibrotic factors and matrix, which initiated the progression of tubulointerstitial fibrosis. Therefore, it suggested that detection of the serum level of TGF-β1, as well as polymorphism of the TGF-β1 gene −509C/T, might provide assistance for evaluating the curative effect and prognosis of PNS, especially for patients who cannot accept renal biopsy due to some contraindication.
In conclusion, these results indicated that the TGF-β1 gene −509C/T polymorphism did not contribute to the genetic predisposition to PNS but was specifically associated with the degree of TID in PNS patients. Patients with the TT genotype were prone to have elevated circulating levels of TGF-β1 and severe TID, and T allele might be an important hereditary factor related to TID in PNS. Because of small sample size, different disease courses, and differences in ethnicity, observations of the association of the TGF-β1 gene −509C/T polymorphism with kidney disease have been contradictory in different clinical reports. A large case number, prospective randomized, controlled, and multicentric study with long-term observation is needed to establish the prognostic significance of this gene polymorphism. We believe that detection of TGF-β1 gene −509C/T polymorphism may be helpful for predicting the degree of TID and progression of proteinuric nephropathy in future clinical studies.
Acknowledgments
This study supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No: 20070533062), a Project by Scientific Research Fund of Hunan Provincial technology Department(Grant No: 2007SK3040) and a project by the Scientific project of Research Center of Metabolic Syndrome in Central South University. (Grant No: DY-2008-02-03).
Declaration of Interest: We declare that the results presented in this paper have not been published previously in whole or part.
REFERENCES
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25.
- Zoja C, Garcia PB, Remuzzi G. The role of chemokines in progressive renal disease. Front Biosci. 2009;14:1815–1822.
- Liu FY, Li Y, Peng YM, Relationship between clinical predictors and tubulointerstitial damage in adult-onset primary nephrotic syndrome. Arch Med Res. 2006;37(8): 981–986.
- Murphy M, Docherty NG, Griffin B, IHG-1 amplifies TGF-beta1 signaling and is increased in renal fibrosis. J Am Soc Nephrol. 2008;19(9):1672–1680.
- Diwakar R, Pearson AL, Colville-Nash P, The role played by endocytosis in albumin-induced secretion of TGF-beta1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;292(5):F1464–F1470.
- Grainger DJ, Heathcote K, Chiano M, Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet. 1999;8:93–97
- Babel N, Gabdrakhmanova L, Hammer MH, Predictive value of cytokine gene polymorphisms for the development of end-stage renal disease. J Nephrol. 2006;19(6):802–807.
- Khalil MS, El Nahas AM, Blakemore AI. Transforming growth factor-beta1 SNPs: Genetic and phenotypic correlations in progressive kidney insufficiency. Nephron Exp Nephrol. 2005;101(2):e31–e41.
- Yamada Y, Miyauchi A, Takagi Y, Association of the C-509T polymorphism, alone of in combination with the T869C polymorphism, of the transforming growth factor-beta1 gene with bone mineral density and genetic susceptibility to osteoporosis in Japanese women. J Mol Med. 2001;79:149–156.
- Gong WK, Cheung W, Yap HK. Minimal change nephrotic syndrome–a complex genetic disorder. Ann Acad Med Singap. 2000;29(3):351–356.
- Bakr AM, El-Chenawi F, Al-Husseni F. HLA alleles in frequently relapsing steroid-dependent and – resistant nephrotic syndrome in Egyptian children. Pediatr Nephrol. 2005;20(2): 159–162.
- Obeidová H, Merta M, Reiterová J, Genetic basis of nephrotic syndrome. Prague Med Rep. 2006;107(1):5–16.
- Hinkes BG, Mucha B, Vlangos CN, Nephrotic syndrome in the first year of life: Two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics. 2007;119(4):e907–e919.
- de Borst MH, Benigni A, Remuzzi G. Primer: Strategies for identifying genes involved in renal disease. Nat Clin Pract Nephrol. 2008;4(5):265–276.
- Liu FY, Li Y, Peng YM, Norcantharidin ameliorates proteinuria, associated tubulointerstitial inflammation and fibrosis in protein overload nephropathy. Am J Nephrol. 2008;28(3): 465–477.
- Hewitson TD. Renal tubulointerstitial fibrosis: Common but never simple. Am J Physiol Renal Physiol. 2009;296(6):F1239–F1244.
- Zhao J, Tramontano A, Makker SP. Albumin-stimulated TGFbeta-1 in renal tubular cells is associated with activation of MAP kinase. Int Urol Nephrol. 2007;39(4):1265–1271
- Lee SB, Kanasaki K, Kalluri R. Circulating TGF-beta1 as a reliable biomarker for chronic kidney disease progression in the African-American population. Kidney Int. 2009 Jul;76(1):10–12.
- Solari V, Owen D, Puri P. Association of transforming growth factor-beta1 gene polymorphism with reflux nephropathy. J Urol. 2005;174(4 Pt 2):1609–1611.
- Lim CS, Kim YS, Chae DW, Association of C-509T and T869C polymorphisms of transforming growth factor-beta1 gene with susceptibility to and progression of IgA nephropathy. Clin Nephrol. 2005;63(2):61–67.
- Cho JH, Huh S, Kwon TG, Association of C-509T and T869C polymorphisms of transforming growth factor-beta1 gene with chronic allograft nephropathy and graft survival in Korean renal transplant recipients. Transplant Proc. 2008;40(7):2355–2360.
- Carturan S, Roccatello D, Menegatti E, Association between transforming growth factor beta1 gene polymorphisms and IgA nephropathy. J Nephrol. 2004;17(6): 786–793.
- Sato F, Narita I, Goto S, Transforming growth factor-beta1 gene polymorphism modifies the histological and clinical manifestations in Japanese patients with IgA nephropathy. Tissue Antigens. 2004;64(1):35–42.