Abstract
The aim of this study was designed to investigate the possible beneficial effects of the angiotensin (ang) II T1 (AT1) receptor blocker, irbesartan (Irb), and the alpha lipoic acid (ALA) in streptozotocin (STZ)-induced diabetic nephropathy (DNP) in rats. The rats were randomly allotted into one of five experimental groups: A, control; B, diabetic untreated; C, diabetic treated with Irb; D, diabetic treated with ALA; and E, diabetic treated with Irb + ALA; each group contains 10 animals. B, C, D, and E groups received STZ. Diabetes was induced in four groups by a single intraperitoneal injection of STZ (50 mg/kg, freshly dissolved in 5 mmol/L citrate buffer, pH 4.5). The rats in Irb-, ALA-, and Irb + ALA-treated groups were given Irb (5 mg/kg), ALA (in a dose of 3 mg/kg), and Irb + ALA (in a dose of 2.5 + 1.5 mg/kg) once a day orally by using intragastric intubation for 12 weeks starting 2 days after STZ injection, respectively. Treatment with ALA and especially Irb reduced the glomerular size; thickening of capsular, glomerular, and tubular basement membranes; increased amounts of mesangial matrix and tubular dilatation as compared with diabetic-untreated rats. Notably, the better effects were obtained when Irb and ALA were given together. We conclude that Irb, ALA, and especially Irb + ALA therapy causes renal morphologic improvement after STZ-induced diabetes in rats. We believe that further preclinical research into the utility of Irb and ALA treatment, alone or its combination, may indicate its usefulness as a potential treatment in DNP.
INTRODUCTION
Diabetic nephropathy (DNP) is characterized by changes in both glomerular and tubular structure and function. Most studies have focused on alterations in the glomerulus, including abnormalities in glomerular permeability and capillary pressure, glomerular hyperplasia or hypertrophy, and increase in mesangial volume.Citation1–3
Irbesartan (Irb) is a newly approved product of angiotensin (ang) II T1 (AT1) receptor blocker with higher bioavailability, lower plasma protein binding, and longer half-life than losartan and valsartan. Irb exerted a renal protective role independently of its antihypertensive effect.Citation4 Lewis et al. reported a multicenter, randomized, and double-blind study with 1715 cases of type 2 diabetes, clearly showing that Irb was effective in protecting against the progression of nephropathy.Citation5 However, the exact mechanism of Irb on renal protection is still to be clarified.
Alpha lipoic acid (ALA – also known as thioctic acid) was discovered in 1951 as a molecule that assists in acyl-group transfer and as a coenzyme in the Krebs cycle. In the 1980s, the scientific community realized that ALA is a powerful antioxidant. Several qualities distinguish ALA from other antioxidants: ALA can be synthesized by animals and humansCitation6; it neutralizes free radicals in both the fatty and the watery regions of cells, in contrast to vitamin C (water soluble) and vitamin E (fat soluble); and ALA functions as an antioxidant in both its reduced and oxidized forms.Citation7
One animal study suggested that ALA may be effective in the prevention of early diabetic glomerular injury and may provide more protection than high doses of vitamin C or vitamin E. The study observed that ALA (30 mg/kg body weight daily for 2 months) given to diabetic rats either prevented or significantly attenuated increases in urinary albumin excretion, fractional albumin clearance, glomerular volume, glomerular content of immunoreactive transforming growth factor-β (TGF-β1), and collagen α1 to levels no different from non-diabetic controls. In addition, it was found that ALA, but not vitamin C or E, significantly increased renal–cortical glutathione content.Citation8
This study focused on the effect of Irb and ALA treatment alone or its combination on renal hypertrophy, thickening of basement membrane, expression of TGF-β1, and inducible nitric oxide synthase (iNOS) in streptozotocin (STZ)-induced diabetic rats.
MATERIAL AND METHODS
Animals
Fifty healthy female Sprague–Dawley rats, weighing 200–285 g and averaging 6 months old, were utilized in this study. They were housed in Macrolin cages under standard laboratory conditions (light period 7.00 am to 7.00 pm, 21 ± 2°C, relative humidity 55%). The animals were given standard rat pellets (Murat Animal Food Product Co., Ankara, Turkey) and tap water ad libitum.
Drugs
Irb was purchased from Sanofi-Aventis (Kansas City, MO, USA), ALA was purchased from Sigma (St. Louis, MO, USA), STZ was purchased from Sigma Chemical Company, and all other chemicals and reagents used were of analytical grade.
Experimental design
All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
The rats were randomly allotted into one of five experimental groups: A, control; B, diabetic untreated; C, diabetic treated with Irb; D, diabetic treated with ALA; and E, diabetic treated with Irb + ALA; each group contains 10 animals. B, C, D, and E groups received STZ (Sigma). Diabetes was induced in four groups by a single intraperitoneal (i.p.) injection of STZ (50 mg/kg, freshly dissolved in 5 mmol/L citrate buffer, pH 4.5).Citation9,Citation10 Two days after STZ treatment, development of diabetes in four experimental groups was confirmed by measuring blood glucose levels in tail vein blood samples. Rats with blood glucose levels of 250 mg/dL or higher were considered to be diabetic. Diabetes mellitus (DM) was confirmed by Ames One Touch Glucometer (LifeScan, Johnson and Johnson, New Brunswick, NJ, USA). Control rats were injected with the same volume of isotonic NaCl as the diabetic animals that received STZ.
The rats in Irb-, ALA-, and Irb + ALA-treated groups were given Irb (5 mg/kg body weight), ALA (in a dose of 3 mg/kg body weight), and Irb + ALA (in a dose of 2.5 + 1.5 mg/kg body weight) once a day orally by using intragastric intubation for 12 weeks starting 2 days after STZ injection. Control and diabetic-untreated rats were received with the same volume of isotonic NaCl as the diabetic-treated animals. The initial and final body weight changes of the various groups were recorded. They were sacrificed and both kidneys were harvested for histopathological investigation.
Histopathologic evaluation
The kidney specimens were individually immersed in Bouin's fixative, dehydrated in alcohol, and embedded in paraffin. Sections of 5 μm were obtained, deparaffinized, and stained with periodic acid Schiff (PAS). The kidney tissue was examined and evaluated in random order under blindfold conditions with standard light microscopy.
Immunohistochemistry
The harvested kidney tissues were fixed in Bouin's fixative, embedded in paraffin, and sectioned at 5 μm thickness. Immunocytochemical reactions were performed according to the ABC technique described by Hsu et al.Citation11 The procedure involved the following steps: (1) endogenous peroxidase activity was inhibited by 3% H2O2 in distilled water for 30 min; (2) the sections were washed in distilled water for 10 min; (3) nonspecific binding of antibodies was blocked by incubation with normal goat serum (Code. X 0907 DAKO; Carpinteria, CA, USA) with PBS, diluted 1:4; (4) the sections were incubated with specific rabbit polyclonal anti-iNOS antibody (Cat. # RB-1605-P; Neomarkers, CA, USA) and rabbit polyclonal anti-TGF-β1 antibody (Sc-146, Lot # 12006; Santa Cruz Biotechnology, CA, USA), diluted 1:50 for 1 hour at room temperature; (5) the sections were washed in PBS thrice for 3 min; (6) the sections were incubated with biotinylated anti-mouse IgG (DAKO LSAB 2 Kit); (7) the sections were washed in PBS thrice for 3 min; (8) the sections were incubated with ABC complex (DAKO LSAB 2 Kit); (9) the sections were washed in PBS thrice for 3 min; (10) peroxidase was detected with diaminobenzidine as substrate; (11) the sections were washed in tap water for 10 min and then dehydrated; (12) the nuclei were stained with hematoxylin; and (13) the sections were mounted in DAKO paramount. All dilutions and thorough washes between steps were performed using phosphate-buffered saline unless otherwise specified. All steps were carried out at room temperature unless otherwise specified. As a negative control, primary antibody was replaced with PBS.
The positive immunostaining of TGF-β1 was scored in a semiquantitative manner in order to determine the differences between the control group and the experimental groups in the distribution patterns of intensity of immunolabeling of kidney tissue. The intensity of the positive staining was recorded as weak (±), mild (+), moderate (++), strong (+++), and very strong (++++). This analysis was performed in at least eight areas per kidney section, in two sections from each animal at ×400 magnification. The intensities of immunostaining of TGF-β1 as detected semiquantitatively are shown in .
TABLE 1. Semiquantitative comparison of the intensity of TGF-β1 in kidney tissues for each group
iNOS-positive cell numbers were scored in a semiquantitative manner in order to determine the differences between the control group and the experimental groups in kidney tissue. The number of positive staining was recorded as absence (–), a few (±), few (+), medium (++), high (+++), and very high (++++). This analysis was performed in at least eight areas per kidney section, in two sections from each animal at ×400 magnification. The numbers of the positive staining of iNOS as detected semiquantitatively are shown in .
TABLE 2. Semiquantitative comparison of the iNOS positive cell numbers in kidney tissues for each group
RESULTS
Histopathological findings
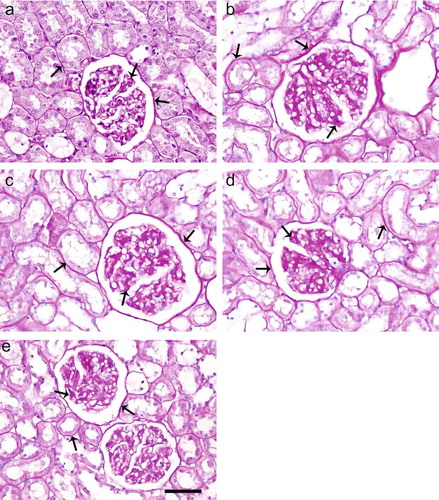
A significant enlargement of the glomeruli; thickening of capsular basement membranes (CBMs), glomerular basement membranes (GBMs), and tubular basement membranes (TBMs); increased amounts of mesangial matrix and tubular dilatation were observed in diabetic-untreated rats (see ). The renal histology in untreated diabetic rats showed accelerated mesangial expansion, thickening of CBMs, GBMs, and TBMs, characterized by an increase in PAS-positive area as compared with control animals (see and b). Treatment of ALA and especially Irb reduced the glomerular size; thickening of CBMs, GBMs, and TBMs; and increased amounts of mesangial matrix and tubular dilatation as compared with diabetics untreated (see and d). ALA treatments in combination with Irb were more effective than ALA or Irb treatment alone (see ).
FIGURE 1. PAS staining of kidney sections of control (a), diabetic untreated (b), diabetic treated with Irb (c), diabetic treated with ALA (d), and diabetic treated with Irb+ALA (e) rats. Increased mesangial matrix, thickened CBMs, TBMs, and GBMs are present in the glomerulus of diabetic untreated rats as compared with the control and diabetic treated rats (arrows: PAS positive area, PAS; scale bar, 50 μm).
Immunohistochemical findings
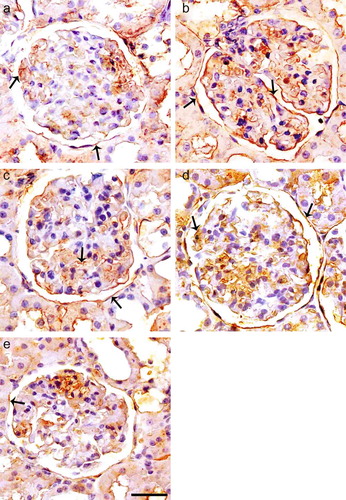
Expression of TGF-β1 was mainly present in CBMs, GBMs, TBMs, and mesangial matrix in diabetic kidney (see ), whereas there was no marked expression of TGF-β1 in the normal kidney. Expression of TGF-β1 could be detected in both glomeruli and tubuli area in normal rat kidney (see ). Semi-quantitative assessment of the immunostaining for TGF-β1 showed that capsular, glomerular, and tubular expression and TGF-β1 in untreated diabetics group were significantly increased as compared with control rats, and the expression of TGF-β1 was lower in ALA and especially Irb-treated diabetic groups as compared with untreated diabetics group (see and d). The expression of TGF-β1 was much lower in ALA treatments in combination with Irb diabetic groups as compared with untreated diabetics group (see and ).
FIGURE 2. Immunoperoxidase staining of TGF-β1 in glomeruli of control (a), diabetic untreated (b), diabetic treated with Irb (c), diabetic treated with ALA (d), and diabetic treated with Irb + ALA (e) rats. Immunostaining of TGF-β1 is increased in glomeruli and tubuli of diabetic rats as compared with the control rats. Immunostaining of TGF-β1 is decreased in glomeruli and tubuli of diabetic-treated rats as compared with the diabetic untreated rats (arrows: positive immunostaining for TGF-β1, immunoperoxidase, hematoxylin counterstain; scale bar, 25 μm).
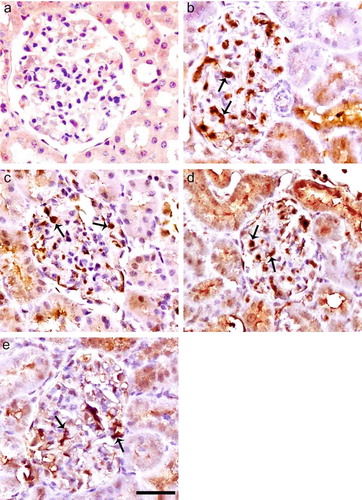
Immunohistochemical staining for iNOS is negative in glomerulus of control rats (see ) but positive in the mesangium, podocytes, and capillary loop of untreated diabetic rats (see ). The expression of iNOS in glomerules was lower in ALA and especially in Irb-treated diabetic groups as compared with untreated diabetic rats (see and d). The expression of iNOS was much lower in ALA treatments in combination with Irb diabetic groups as compared with untreated diabetics group (see and ).
FIGURE 3. Immunoperoxidase staining of iNOS in glomeruli of control (a), diabetic untreated (b), diabetic treated with Irb (c), diabetic treated with ALA (d), and diabetic treated with Irb + ALA (e) rats. Immunohistochemical staining for iNOS is negative in glomerulus of control rats, but positive in the mesangium, podocytes, and capillary loop of untreated diabetic rats. The expression of iNOS in glomerules was lower in ALA and especially Irb-treated diabetic groups as compared with untreated diabetic rats. The expression of iNOS was much lower in ALA treatments in combination with Irb diabetic groups as compared with untreated diabetic group (arrows: positive immunostaining for iNOS, immunoperoxidase, hematoxylin counterstain; scale bar, 25 μm).
DISCUSSION
DM develops microvascular complications including DNP and has emerged as a leading cause of end-stage renal disease.Citation12 DNP is characterized clinically by proteinuria and histologically by GMBs thickening and mesangial matrix expansion, resulting in glomerulosclerosis.Citation13 TGF-β1 is a ubiquitously expressed multifunctional cytokine critical to development and wound healing.Citation14,Citation15 In fact, TGF-β1 is known as a key mediator of sclerosing process in diseased glomeruli and is related to glomerulosclerosis and interstitial fibrosis in various renal diseases.Citation16–19 TGF-β1 is increased in kidney as well as serum of diabetic animals and patients, demonstrating possible contribution of TGF-β1 to DNP.Citation20–23
DNP, one of the major complications of DM, often leads to end-stage renal failure. Glomerular hypertrophy and progressive expansion of extracellular matrix have been regarded as the early features of DNP, which lead to the accumulation of extracellular matrix and thickening of GBMs, and subsequently induce renal fibrosis.Citation24,Citation25
However, the precise mechanism of DNP has not been fully understood. Recently, considerable evidence suggests that the intrarenal renin–angiotensin–aldosterone system (RAAS) plays an important role in the development of DNP.Citation26 Blockade the RAAS with either ACE inhibitor or ang II AT1 receptor antagonist delays the progression of renal injury associated with diabetes.Citation27,Citation28
Irb is a newly approved product of AT1 receptor blocker with higher bioavailability, lower plasma protein binding, and longer half-life than losartan and valsartan. Irb exerted a renal protective role independent of its antihypertensive effect.Citation4
In this study, we evaluated whether Irb, ALA, and especially Irb + ALA therapy causes renal morphologic improvement after STZ-induced diabetes in rats. Similar protective effects were seen in all treatment groups in renal functional assessment. ALA slows down the histopathologic alterations and deterioration. Irb corrected the histopathologic alterations more prominently than ALA. In attenuating the progression of glomerulosclerosis, the combination therapy with ALA and Irb was found more effective than ALA or Irb alone.
Several studies have documented the expression of the renal ang receptors. Vasoconstriction, stimulation of aldosterone release as well as most of tissue effects of ang II, including growth, fibrosis, thrombosis, inflammation, and oxidation, are mediated by the AT1 receptors.Citation29 AT2 receptors generally oppose the actions of AT1 receptors, mediating various antiproliferative and anti-inflammatory effects and promoting tissue differentiation and regeneration and apoptosis.Citation30
ALA, a naturally occurring short chain fatty acid with sulfhydryl group, is an essential cofactor of mitochondrial respiratory enzymes and has potent antioxidative capacity.Citation7 ALA improves insulin sensitivity and stimulates peripheral glucose utilization, resulting in the prevention of development of DM in rats.Citation31–33 In DM, ALA reduces urinary albumin excretion, fraction clearance of albumin, glomerular volume, glomerular content of TGF-β1, and glomerular mesangial matrix expansion, and inhibits progression of albuminuria.Citation8,Citation34–36 Melhem et al.Citation8,Citation34 have described that ALA prevented the elevation of urinary albumin excretion, fraction clearance of albumin, glomerular volume, glomerular content of TGF-β1, and glomerular mesangial matrix expansion in DM. The data reported by Morcos et al.Citation35 have shown that ALA inhibited the progression of albuminuria in diabetic patients. It has been revealed that the expression of TGF-β1 was increased in renal cortex and that ALA attenuated the increased expression of glomerular TGF-β1, resulting in improvement of glomerular injury in cultured mesangial cells and STZ-induced diabetic rats.Citation21,Citation36,Citation37 In our study, it has been shown that the expression of TGF-β1 was much lower in ALA treatments in combination with Irb diabetic groups as compared with untreated diabetics group.
A number of studies have examined renal NOS expression in diabetes. In the early phase of diabetes, iNOS expression was unchangedCitation38 or barely detectable.Citation39 However, Jeong et al.Citation40 speculate that sildenafil may decrease the generation of peroxynitrite by downregulating iNOS expression in STZ-induced diabetic rat kidneys. In our study, it has been shown that the expression of iNOS was much lower in ALA treatments in combination with Irb diabetic groups as compared with untreated diabetic group.
We conclude that Irb, ALA, and especially Irb + ALA therapy causes renal morphologic improvement after STZ-induced diabetes in rats. We believe that further preclinical research into the utility of Irb and ALA treatment, alone or its combination, may indicate its usefulness as a potential treatment in DNP.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rohrbach DH, Wagner CW, Star VL, Martin GR, Brown KS, Yoon JW. Reduced synthesis of basement membrane heparan sulfate proteoglycan in streptozotocin induced diabetic mice. J Biol Chem. 1983;258:11672–11677.
- Ihm CG, Lee GS, Nast CC, Early increased renal procollagen alpha 1(IV) mRNA levels in streptozotocin induced diabetes. Kidney Int. 1992;41:768–777.
- Reddi AS, Nimmagadda VR, Arora R. Effect of antihypertensive therapy on renal artery structure in type 2 diabetic rats with hypertension. Hypertension. 2001;37:1273–1278.
- Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878.
- Lewis EJ, Hunsicker LG, Clarke WR, Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860.
- Carreau JP. Biosynthesis of lipoic acid via unsaturated fatty acids. Methods Enzymol. 1979;62:152–158.
- Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250.
- Melhem MF, Craven PA, De Rubertis FR. Effects of dietary supplementation of α-lipoic acid on early glomerular injury in diabetes. J Am Soc Nephrol. 2001;12:124–133.
- Kanter M, Meral I, Yener Z, Ozbek H, Demir H. Partial regeneration/proliferation of the ß-cells in the islets of Langerhans by Nigella sativa L. in streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2003;201:213–219.
- Kanter M, Coskun O, Korkmaz A, Oter S. Effects of Nigella sativa on oxidative stress and ß-cell damage in streptozotocin-induced diabetic rats. Anat Rec. 2004;279:685–691.
- Hsu SM, Raine L, Fanger H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580.
- Collart F. Kidney failure, proteinuria, and diabetic nephropathy. Rev Med Brux. 2003;24:A257–A262.
- Kreisberg JI, Garoni JA, Radnik R, Ayo SH. High glucose and TGFβ1 stimulate fibronectin gene expression through a cAMP response element. Kidney Int. 1994;46:1019–1024.
- Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-β superfamily. Cytokine Growth Factor Rev. 1996;7:327–339.
- August P, Suthanthiran M. Transforming growth factor β signaling, vascular remodeling, and hypertension. N Engl J Med. 2006;354:2721–2723.
- Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292.
- Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells: A possible role in collagen expression. Kidney Int. 1999;56: 1710–1720.
- Border WA, Noble NA. Cytokines in kidney disease: The role of transforming growth factor β. Am J Kidney Dis. 1993;22: 105–113.
- Poncelet AC, Schnaper HW. Regulation of human mesangial cell collagen expression by transforming growth factor-β1. Am J Physiol. 1998;275:F458–F466.
- Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90:1814–1818.
- Yagi K, Kim S, Wanibuchi H, Yamashita T, Yamamura Y, Iwao H. Characteristics of diabetes, blood pressure, and cardiac and renal complications in Otsuka Long-Evans Tokushima fatty rats. Hypertension. 1997;29:728–735.
- Shankland SJ, Scholey JW. Expression of transforming growth factor β1 during diabetic renal hypertrophy. Kidney Int. 1994;46:430–442.
- Hellmich B, Schellner M, Schatz H, Pfeiffer A. Activation of transforming growth factor-β1 in diabetic kidney disease. Metabolism. 2000;49:353–359.
- Kreisberg JI, Ayo SH. The glomerular mesangium in diabetes mellitus. Kidney Int. 1993;43:109.
- Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637.
- Leehey DJ, Singh AK, Alavi N, Singh R. Role of angiotensin II in diabetic nephropathy. Kidney Int. 2000;58:S93–S98.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462.
- Cao Z, Bonnet F, Davis B, Allen TJ, Cooper ME. Additive hypotensive and anti-albuminuric effects of angiotensin-coverting enzyme inhibition and angiotensin receptor antagonist in diabetic spontaneously hypertensive rats. Clin Sci. 2001;100:591–599.
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287.
- Steckelings UM, Kaschina E, Unger T. The AT2 receptor – a matter of love and hate. Peptides. 2005;26:1401–1409.
- Song KH, Lee WJ, Koh JM, Alpha-lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem Biophys Res Commun. 2005;326:197–202.
- Lee WJ, Song KH, Koh EH, Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891.
- Kim MS, Park JY, Namkoong C, Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733.
- Melhem MF, Craven PA, Liachenko J, DeRubertis FR. Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol. 2002;13:108–116.
- Morcos M, Borcea V, Isermann B, Effect of α-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: An exploratory study. Diabetes Res Clin Pract. 2001;52:175–183.
- Budisavljevic MN, Hodge L, Barber K, Oxidative stress in the pathogenesis of experimental mesangial proliferative glomerulonephritis. Am J Physiol Renal Physiol. 2003;285: F1138–F1148.
- Oksala NK, Lappalainen J, Laaksonen DE, Alpha-lipoic acid modulates heat shock factor-1 expression in streptozotocin-induced diabetic rat kidney. Antioxid Redox Signal. 2007; 9:497–506.
- Ishii N, Patel KP, Lane PH, Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol. 2001;12(8):1630–1639.
- Veelken R, Hilgers KF, Hartner A, Haas A, Bohmer KP, Sterzel RB. Nitric oxide synthase isoforms and glomerular hyperfiltration in early diabetic nephropathy. J Am Soc Nephrol. 2000;11(1):71–79.
- Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29:274–282.
