Abstract
Background: Kidney transplantation is the treatment of choice for end-stage renal disease that restores the patients' quality of life and reduces the morbidity and mortality rates induced by renal failure and its complications. However, after transplantation the organ and patient survival rates are important issues of interest in many centers worldwide. Subjects and methods: This is a historical cohort study planned to determine the organ survival rate after kidney transplantation from deceased donor during a period of 10 years (March 1999–March 2009) in Shiraz Transplant Center, Namazi Hospital, Shiraz, Iran. We tried to clarify the probable contributory risk factors implicating in graft loss. Kaplan–Meier method was used to determine the survival rate. Log-rank test was used to compare survival curves, and Cox regression model to define the hazard ratio and for modeling of factors implicating in survival rate. Results: Mean follow-up period was 37.54 ± 28.6 months. Allograft survival rates at 1, 3, 5, and 9 years after kidney transplantation from deceased donor (calculated by Kaplan–Meier method) was found to be 93.7, 89.1, 82.1, and 80.1%, respectively. Duration of dialysis before operation and creatinine level at discharge were showed to be the most important factors influencing survival rate of renal allograft. Conclusion: Overall long-term graft survival in our cohort is satisfactory and comparable with reports from large centers in the world. Duration of dialysis before operation and creatinine level at discharge are the only independent factors that could correlate with long-term graft survival in our cohort.
INTRODUCTION
According to the most recent report published by Management Center for Transplantation and Special Disease affiliated to Iran's Ministry of Health in 2006, there were 25,000 patients with end-stage renal disease (ESRD) who were under renal-replacement therapy (RRT) in Iran (population = 70 million people). Considering an annual growing rate of 12%, the number can reach 40,000 patients by the year 2011. The prevalence and incidence of ESRD in Iran have been reported to be 357 and 57 cases per 1 million populations per year, respectively.Citation1 Most of these patients have been treated by regular hemodialysis, peritoneal dialysis, and/or renal transplantation.Citation2–5 During the recent years, RRT has been increasingly prescribed in the entire world, which is not accepted for Iran.Citation1 Kidney transplantation is the treatment of choice for ESRDCitation6,Citation7 that restores the patients' quality of life and reduces the morbidity and mortality rates induced by renal failure and its complications.Citation3,Citation8,Citation9 At present, the rate of renal transplantation in Iran approximates 24 grafts per 1 million populations per year.Citation10 Although there are three different resources for organ attainment, including live related, live unrelated, and deceased donors, organ shortage is still the biggest obstacle restricting proper transplantation for every patient who needs it.Citation11 The kidneys of deceased donors are the most commonly accepted source of organ attainment for transplantation in patients with ESRD.Citation12 Allograft rejection is the most important complication that limits the organ survival after transplantation; however, many factors contribute to allograft survival or rejection.
We designed this study to determine the organ survival rate after kidney transplantation from deceased donor during a period of 10 years (March 1999–March 2009) in Shiraz Transplant Center, Namazi Hospital.
SUBJECTS AND METHODS
This is a historical cohort study designed to assess the organ survival rate and its contributory factors in 512 patients who received kidney transplantation from deceased donor, in Shiraz Transplant Center, Namazi Hospital, during a period of 10 years: from March 1999 to March 2009. The exact time of transplantation was considered to be the “initial event,” and when renal allograft was diagnosed to be completely and irreversibly non-functioning due to any cause including rejection and the patient needs regular dialysis again, it was defined as “end-point event.” As a rule, we do not use donors whose last preoperative serum creatinine level was over 3 mg/dL or last 24 hours urine output was less than 1 mL/kg/hr.
All needed data were collected through reviewing of patients' hospital records. The organ survival and every patient's need to regular dialysis were assessed and determined by nephrologists and recorded in follow-up clinics and related institutions such as Management Center for Transplantation and Special Diseases and Renal Patients Support Society. The studied variables include donor's and recipient' age, gender, blood group, recipient's immunosuppressive drug regimen, underlying cause of ESRD, time of first urination, vascular complications, endarterectomy, allograft warm and cold ischemic times, creatinine level at discharge, and the duration of dialysis therapy before and hospital stay after transplantation.
We use intravenous methylprednisolone for induction of immunosuppressive regimen for all patients. Four different regimens had been prescribed to recipients for the maintenance of immunosuppressive regimen:
Oral prednisolone, azathioprine (Imuran®, GlaxoSmithKline, Brentford, UK), and cyclosporine (Neoral®, Novartis, Basel, Switzerland);
Oral prednisolone, mycophenolate mofetil (Cellcept®, Roche, Switzerland), and cyclosporine (Neoral®);
Oral prednisolone, azathioprine, which was changed to mycophenolate mofetil (Cellcept®) after different time intervals, and cyclosporine (Neoral®);
Oral prednisolone, mycophenolate mofetil (Cellcept®), and tacrolimus (Prograf®, Astellas Pharma, Deerfield, IL, USA).
Allograft survival rate was calculated by Kaplan–Meier method at 1, 3, 5, and 9 years after kidney transplantation. Log-rank test was used to compare survival curves and Cox regression models to define the hazard ratio and for modeling of factors implicating the survival rate. The SPSS® software (version 15) was used for statistical analysis of data. The P-value of less than 0.05 was considered to be statistically significant.
RESULTS
The total number of kidney transplantation recipients in Shiraz Transplant Center, Namazi Hospital, during a period of 10 years, from March 1999 to March 2009, reached 1356 allografts, which consisted of 403 (29.7%) kidneys from living related donors, 441 (32.5%) kidneys from living unrelated donors, and 512 (37.8%) kidneys from deceased donors. We could follow 487 (95.1%) out of the remaining 512 patients who had received kidney from deceased donors and found that 59 (12.1%) of them had been eventuated in irreversibly non-functioning allografts that some of them necessitated regular dialysis again and some cases died. The mean age of kidney donors and recipients were 29.22 ± 14.39 (range: 2–78) and 33.77 ± 14.72 (range: 4–73) years, mostly in the age group of 21–40 years that included 43.9% of donors and 45.3% of recipients. The mean length of hospital stay was 12.38 ± 6.26 (range: 3–46) days. Mean follow-up period was 37.54 ± 28.6 months.
As mentioned in , there was male preponderance among both recipients and donors, 57.1 and 73.1% of them were male, respectively. The most common blood group among both donors and recipients was blood group O, comprising 47.6 and 45.2% of cases, respectively. The underlying cause of ESRD was mostly unknown (comprising 57.8% of cases). However, the most common diagnosed renal disease leading to ESRD was revealed to be glomerulonephritis (in 13.3% of cases).
TABLE 1. Frequency distribution of studied variables compared with each other and their related survival rates in different time intervals including 1, 3, 5, and 9 years after kidney transplantation
As shown in , the second regimen was the most frequently used immunosuppressive therapy that had been used in nearly 63% of cases. Fourth regimen was used for patients with panel reactive antibody >20% or patients with second transplantation due to immunologic rejection of their previous grafts. However, the effects of third and fourth drug-regimens on graft survival were not assessed because of their rare administration, too infrequently to be included in statistical analysis.
TABLE 2. Frequency distribution of surgical/medical variables during and after operation compared with each other and their related survival rates in different time intervals including 1, 3, 5, and 9 years after kidney transplantation
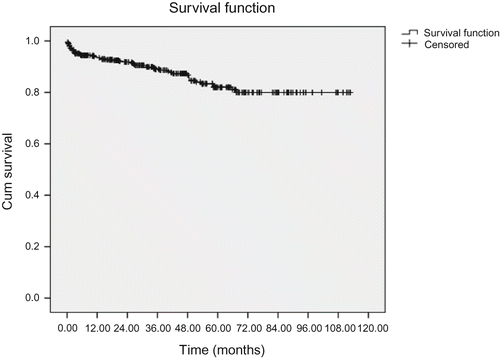
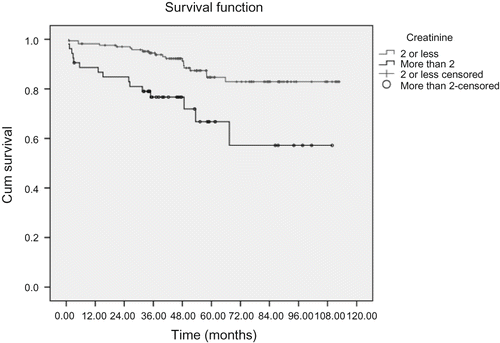
shows allograft survival rate at 1, 3, 5, and 9 years after kidney transplantation and was revealed to be 93.7, 89.1, 82.1, and 80.1%, respectively. Allograft survival rate as mentioned in and are determined for both total studied patients and each contributing variable separately. However, the contributing variables that have been mentioned in make no significant difference in related survival rates as showed by P-values that has been calculated by log-rank test. Univariate analysis revealed that post-operative hospital stay has a meaningful correlation with organ survival rate (P = 0.004). As shown in , organ survival rate has been significantly higher in those patients who had been hospitalized for 7–14 days after renal transplantation. As shown in and , our study revealed that the creatinine level at discharge has a statistically significant relation with organ survival rate (P = 0.001), and univariate analysis demonstrate that cold ischemia time was another factor that has a statistical relation with graft survival rate (P = 0.006).
FIGURE 2. Allograft survival rate in renal transplant recipients compared with the creatinine level at discharge after transplantation.
Those parameters that were assessed by log-rank test as a univariate analysis method showed to have a P-value of less than 0.25 and were tested by Cox regression model (using forward stepwise method). As shown in , the following independent variables were found to have a meaningful association with higher survival rates:
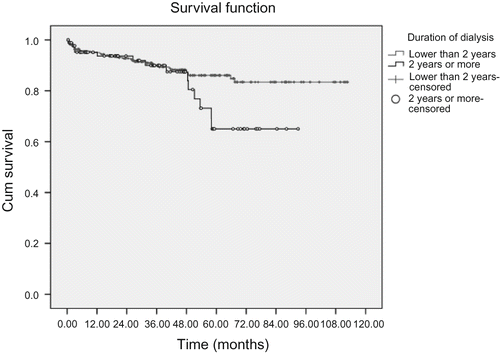
Duration of dialysis before renal transplantation (lower than 2 years);
The creatinine level at discharge (2 mg/dL or less).
TABLE 3. Multivariate analysis by Cox regression model
As shown in , using Cox regression model, our study revealed that the duration of dialysis before operation had been related with graft survival rate, and the hazard ratio was 3.14 in those patients who had dialysis time before operation for more than 2 years (95% CI = 1.37–7.16, P = 0.007). And creatinine level at discharge had an inverse relationship with graft survival as the hazard ratio was 3.23 (95% CI = 1.42–7.31, P = 0.005) for the creatinine level of more than 2 mg/dL, if compared with those of 2 mg/dL or lower.
DISCUSSION
Although living donor kidney transplantation is superior to deceased-donor renal transplantation, a critical shortage of donor organs leads to the expanding use of deceased donors in oriental countries.Citation13 Many factors have been depicted in the literature as influencing factors of graft survival in kidney transplant recipients. These include recipient and donor age,Citation14 type of the donor (living related, living unrelated, or deceased donor), underlying cause of ESRD (e.g., diabetes mellitus, focal segmental glomerulonephritis,Citation15 oxalosis), number and time of acute rejection episodes,Citation16 chronic allograft nephropathy,Citation17 percent of panel reactivity,Citation13 human leukocyte antigen (HLA) mismatch,Citation18 delayed graft function,Citation19 hyperlipidemia and hypertension,Citation20,Citation21 post-transplant hospitalization, recipient size,Citation22 type of immunosuppressive regimen,Citation23 cause of brain death (hypertensive stroke victims vs. others),Citation24 and so on. Because of retrospective nature of our study in this historical cohort we could not take into account all of these factors, and only those factors that were reachable from patients medical record were used for data analysis.
As shown in , male gender is predominant in both donors and recipients in our cohort, but there was no significant difference in survival of the patients. Also there is no significant correlation between the recipient and donor age, duration of dialysis(less than 2 years vs. over 2 years), cause of ESRD (diabetic vs. non-diabetic, known vs. unknown), blood group (compatible vs. identical), and graft survival of our patients.
In many of the reports in the literature age is a risk factor for graft survival. For example, Pugliese et al. reported from Italy a significant association between donor age and recipient age with graft survival (old vs. young, relative risk of 1.62 and 1.25, respectively).Citation25 Truan Cacho et al. reported the same effect of ageCitation26 from Spain. As shown by mean age of our donors (29.22 ± 14.39), most of brain death patients in our center are in young age group (95% CI = 14.83–43.61) and the number of old-age donors (>50 Y/O) was only 10.1% of our donors. This might be the reason why age is not a risk factor for decreasing our patients' graft survival.
Wu et al.Citation27 from Pittsburg suggest that diabetes is a major comorbidity of ESRD patients that could compromise graft survival in these patients. In our cohort, diabetes is not a risk factor for reducing graft survival. Again, low number of diabetic patients (5.2%) in our cohort may explain this difference. As shown in , data about the surgical anatomy for the graft (number of artery and veins, use of internal or external iliac artery or vein, or endarterectomy) had no effect on graft survival in our patients. Univariate analysis shows that cold ischemic time is the independent factor that could correlate with long-term graft survival in our cohort, but using Cox regression model we found that this variable was not related to graft survival rate. Detrimental effect of increasing cold ischemia time on long-term graft survival has been proved in several other reports, for example, Giblin et al.Citation28 compared the recipients of a first deceased donor transplanted and the recipient of the second donor kidney transplanted. The 5- and 10-year survival was 72 and 55% for the first kidney transplanted, respectively, compared with 65 and 40% for the second kidney transplanted. Many of the kidneys that were transplanted from deceased donors in our center had cold ischemia time less than 2 hours (35.9%), because our center in Shiraz is one of the most active centers for retrieval of deceased-donor organs and is the main center for liver transplantation in Iran. Most of other centers in Iran have active kidney transplant programs, and kidney grafts are not shipped between these centers because we have no national registry for organ sharing that could prioritize recipients according to their HLA matching or other factors. Interestingly, as depicted earlier, 37.8% of all kidney transplants in our center are from deceased donors and 32.5% are from living unrelated donors. This is in contrary with previous reports of “Iranian model” for kidney transplantationCitation10,Citation29 and we are very proud of this success in decreasing the number of living unrelated kidney transplantation in our center.
Our study shows that in univariate analysis there is no difference in long-term graft survival between patients who are on immunosuppressive maintenance by cyclosporine and mycophenolate mofetil combination compared with cyclosporine and azathioprine combination. In the United States, the frequencies of use of different immunosuppressive agents for kidney transplant recipients in 2006 were as followsCitation30: corticosteroids (68%), tacrolimus (82%), cyclosporine (12%), mycophenolate mofetil (76%), or mycophenolate sodium (12%), or azathioprine (0.9%), sirolimus (8%), or everolimus (0.5%). Because this is a long-term study from over 10 years ago, 24.8% of our patients were under treatment by azathioprine. At present the routine immunosuppressive regimen for all kidney transplant recipients in our center consists of oral prednisolone plus cyclosporine and mycophenolate mofetil, except for those who received their second or more kidney grafts or those with PRA over 20% or with high probability of acute rejections or recurrence of immunologic diseases (such as patients with systemic lupus erythematosus or rapidly progressive glomerulonephritis as the cause of their chronic kidney disease (CKD)). Several initial pivotal studiesCitation31–34 had shown that mycophenolate, despite its increased cost, could decrease acute rejection rates compared with azathioprine. But, given current evidence, azathioprine and mycophenolate mofetil appear to be similar in terms of acute rejection rates and long-term allograft survival rates.Citation35 Our results could be another evidence for this concept.
Cox regression model has shown that length of post-transplant hospitalization (LOH) was not an independent risk factor for reducing graft survival in our cohort. There has been a general trend toward shortened LOH in recent years. In the last decade, the average LOH for kidney transplant patients have decreased from 12.7–19 to 5–7.5 days.Citation36,Citation37 Based on Lin et al. studyCitation37 shorter than 4 days post-kidney transplant hospitalization may potentially be harmful to long-term graft and recipient survival. In our study, best long-term graft survival was for patients with hospital stay between 1 and 3 weeks and those who discharge in the first week or after 2 weeks shows less favorable results. Longer hospital stays in our center; usually is due to a problem with the kidney grafts such as delayed function from any cause (e.g., acute tubular necrosis or severe humeral rejections that need additional treatment modalities such as plasmapheresis or antithymocyte globulin (ATG)). Our routine is to discharge the patients when their serum creatinine level is lower than 2 mg/dL and urine output is around 1 mL/kg/min (∼7–10 days after the operation). In other words, LOH lower than 1 week or greater than 2 weeks naturally correlate with a problem in the kidney function that might translate into lower long-term graft survival.
Relationship of dialysis time before operation and creatinine level at discharge with graft survival rate should be discussed.
In summary, irrespective of these influencing factors, overall long-term graft survival in our cohort is satisfactory and comparable with reports from large centers in the world.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Abbaszadeh S, Nourbala MH, Taheri S, Renal transplantation from deceased donor in Iran. Saudi J Kidney Dis Transpl. 2008;19(4):664–668.
- Abboud O. Incidence, prevalence and treatment of end stage renal disease in the Middle East. Ethn Dis. 2006;16(Suppl. 2): S2-2–S2-4.
- Mahdavi-Mazdeh M, Heidari-Rouchi A, Aghighi M, Organ and tissue transplantation in Iran. Saudi J Kidney Dis Transpl. 2008;19(1):127–131.
- Mahdavi-Mazdeh M, Heidary-Rouchi A, Norozi S, Renal replacement therapy in Iran. Urol J. 2007;4:66–70.
- Schena FP. Epidemiology of end stage renal disease: International comparisons of renal replacement therapy. Kidney Int. 2000;57:39–45.
- Hariharan S, Johnson CP, Barbara A, Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612.
- Cacho DT, Cusi LIP, Pique AA, Elderly donor kidney transplant: Factors involved in graft survival. Transplant Proc. 2005;37:3690–3992.
- Kriesche HUM, Port FK, Ojo AO, Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58:1311–1317.
- Shrestha BM, Haylor JL. Factors influencing long term outcome following renal transplantation: A review. J Nepal Med Assoc. 2007;46(167):136–142.
- Ghods AJ. Renel transplantation in Iran. Nephrol Dial Transplant. 2002;17:222–228.
- Foster CE, Weng RR, Pharm D, The influence of organ acceptance criteria on long term graft survival: Outcome of a kidney transplant program. Am J Surg. 2008;195:149–152.
- Tan SY, Chen TP, Lee SH, Cadaveric renal transplantation at university hospital Kuala Lumpur: A preliminary report. Transplant Proc. 2000;32:1811–1812.
- Cecka JM. The UNOS renal transplant registry. Clin Transplant. 2001;7;1–18.
- Meier-Kriesche HU, Cibrik OM, Ojo AO, Interaction between donor and recipient age in determining the risk of chronic renal allograft failure. J Am Geriatr Soc. 2002;50:14–17.
- Denton MD, Singh AK. Recurrent and de novo glomerulonephritis in the renal allograft. Semin Nephrol. 2000;20:164–175.
- Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: Mechanisms and new approaches to therapy. Semin Nephrol. 2000;20:126–147.
- Ojo AO, Hanson JA, Wolfe RA, Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–313.
- Takemoto SK, Terasaki PI, Gjertson DW, Twelve years’ experience with national sharing of HLA-matched cadaveric kidneys for transplantation. N Engl J Med. 2000;343:1078–1084.
- Kwon OJ, Ha MK, Kwak JY, The impact of delayed graft functions on graft survival in living donor kidney transplantation. Transplant Proc. 2003;35:92–93.
- Mange K, Feldman H. Blood pressure and prediction of patient outcome. Kidney Int. 2000;57:2655.
- Akioka K, Takahara S, Ichikawa S, Factors predicting long-term graft survival after kidney transplantation: Multicenter study in Japan. World J Surg. 2005;29:249–256.
- Meier-Kriesche HU, Arndorfer JA, Kaplan B, The impact of body mass index on renal transplant outcomes: A significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70–74.
- Meier-Kriesche HU, Kaplan B. Cyclosporine microemulsion and tacrolimus are associated with decreased chronic allograft failure and improved long-term graft survival as compared with Sandimmune. Am J Transplant. 2002;2:100–104.
- Port FK, Dykstra DM, Merion RM, Wolfe RA. Trends and results for organ donation and transplantation in the United States 2004. Am J Transplant. 2005;5(4 Pt. 2):843–849.
- Pugliese O, Quintieri F, Mattucci DA, Venettoni S. Kidney graft survival in Italy and factors influencing it. Prog Transplant. 2005;15(4):385–391.
- Truan Cacho D, Peri Cusí LI, Agud Piqué A, Elderly donor kidney transplant: Factors involved in graft survival. Transplant Proc. 2005;37:3690–3692.
- Wu C, Evans I, Joseph R, Comorbid conditions in kidney transplantation: Association with graft and patient survival. J Am Soc Nephrol. 2005;16:3437–3444.
- Giblin L, O’Kelly P, Little D, A comparison of long-term graft survival rates between the first and second donor kidney transplanted–the effect of a longer cold ischemic time for the second kidney. Am J Transplant. 2005;5:1071.
- Einollahi B. Iranian experience with the non-related renal transplantation. Saudi J Kidney Dis Transpl. 2004;15(4): 421–428.
- Meier-Kriesche HU, Li S, Gruessner RW, Immunosuppression: Evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6(5 Pt. 2):1111–1131.
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60:225.
- Shapiro R, Jordan ML, Scantlebury VP, A prospective, randomized trial of tacrolimus/prednisone versus tacrolimus/prednisone/mycophenolate mofetil in renal transplant recipients. Transplantation. 1999;67:411.
- Ojo AO, Meier-Kriesche HU, Hanson JA, Mycophenolate mofetil reduces late renal allograft loss independent of acute rejection. Transplantation. 2000;69:2405.
- Ciancio G, Miller J, Gonwa TA. Review of major clinical trials with mycophenolate mofetil in renal transplantation. Transplantation. 2005;80:S191.
- Remuzzi G, Cravedi P, Costantini M, Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: The MYSS follow-up randomized, controlled clinical trial. J Am Soc Nephrol. 2007;18:1973.
- Shapiro R, Jordan ML, Scantlebury VP, Reducing the length of stay after kidney transplantation – the intensive outpatient unit. Clin Transplant. 1998;12:482.
- Lin S-J, Koford JK, Baird BC, The association between length of post-kidney transplant hospitalization and long-term graft and recipient survival. Clin Transplant. 2006;20:245–252.