Abstract
The nephrotic syndrome is a rare complication of allogeneic stem cell transplantation (alloHSCT). We present two cases of nephrotic syndrome during chronic graft-versus-host disease (GvHD) involving altered cytokine gene expression in renal tissue. A patient with acute lymphatic leukemia demonstrated nephrotic syndrome due to minimal change disease as a marker of chronic GvHD. A patient with acute lymphoblastic leukemia suffered from severe nephrotic syndrome due to membranous glomerulopathy. In the two presented cases of GvHD-linked nephrotic syndrome, increased cytokine gene expression [tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), interferon gamma (IFN-γ), interleukin 2 (IL-2), IL-6, and IL-10] assessed using semiquantitative evaluation with reverse transcriptase polymerase chain reaction (RT-PCR) in situ on renal biopsy was observed.
INTRODUCTION
Involvement of kidneys is occasionally reported as a clinical manifestation of chronic graft-versus-host disease (GvHD).Citation1,Citation2 The nephrotic syndrome due to glomerulonephritis is a rare complication of alloHSCT. It is acknowledged as chronic GvHD-dependent usually as the diagnosis of exclusion.Citation3 The exact pathogenic mechanism is not completely elucidated.
GvHD is a frequent complication of allogeneic stem cell transplantation (alloHSCT) occurring in 70% of the patients. Classically it is subdivided into acute and chronic GvHD forms on the basis of symptomatology and time period post-HSCT when the symptoms develop.
The acute GvHD classically is clinically apparent until day +100 post-HSCT and results from the presence of transplanted donor T-cells which may recognize the recipient's tissues specificities as foreign. The chronic GvHD previously was diagnosed only after day +100 but now the two forms of GvHD are recognized to be a distinct pathological entity which can occur at any time after alloHSCT. Chronic GvHD can last for years, which results at least in part from the allorecognition of recipients' antigens by newly differentiated alloreactive T cells. There are many clinical manifestations of chronic GvHD which can target skin, eye, mucosa, liver, gastrointestinal tract, lung, musculoskeletal as well as nervous and hematopoietic system.
We present two cases of patients with morphologically different glomerulopathies presented by nephrotic syndrome occurring after alloHSCT involving altered cytokine gene expression in renal tissue.
CASE 1
A 30-year-old male (GS) with acute lymphatic leukemia diagnosed in July 2000 underwent alloHSCT in the first remission (15 months after diagnosis). During remission induction and consolidation therapy, he was treated according to the PALG 4–96 protocol (remission induction: daunombicin (DNR), vincristine (VCR), cyclophosphamide (CPD), cytosine-arabinoside (Ara-C), l-asparaginase; consolidation: CPD, Ara-C, methotrexate (MTX), VePesid with addition of Ara-C; maintenance: 6MP as well as central nervous system prophylactic radiotherapy). Remission was documented by blood and bone marrow tests. Transplantation conditioning consisted of busulphan (16 mg/kg), cyclophosphamide (120 mg/kg), and ATG (10 mg/kg).
He was transplanted with peripheral blood progenitor cells (PBPCT) from a matched unrelated donor. Post-transplant GvHD prophylaxis consisted of cyclosporin A. Complications after transplantation encompassed regimen-related toxicity (mostly mucositis and diarrhea), fever with CRP increase, acute GvHD grade II (mostly skin involvement), CMV reactivation, and hemorrhagic cystitis.
Twenty months after transplantation he was admitted to hospital with severe lower extremity edemas and weight gain. Urinary protein expression was 13.43 g/day. Laboratory assays confirmed severe nephrotic syndrome with hypoalbuminemia (15 g/L), hypoproteinemia (36 g/L), hypercholesterolemia (13 mmol/L), and hypertriglyceridemia (7.39 mmol/L). Erythrocyte sedimentation rate was 83 mm/h. Thrombocytopenia 86,000/mL and elevated liver enzymes (AST 60 IU/L, ALT 43 IU/L, GGT 4662 IU/L) were present. Renal filtration was within normal range.
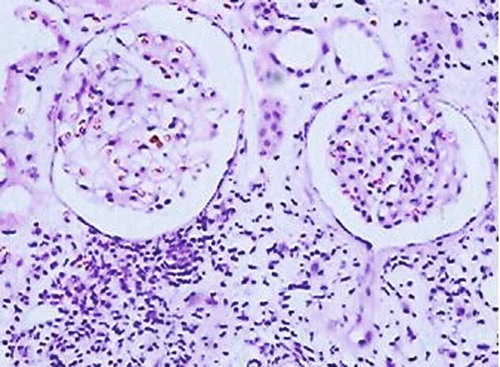
Ultrasonography (USG) examination revealed normal size, slightly hyperechogenic kidneys. Percutaneous kidney biopsy was performed and renal histology demonstrated minimal change disease (MCD) with apparent structurally normal glomeruli with periglomerulitis, non-specific focal areas of tubulointerstitial fibrosis and associated infiltrating lymphocytes CD3+ >80%, CD20+ 5–10%, interstitial fibrosis and tubular atrophy. Immunohistochemical studies were negative as is typical for MCD: no IgA or IgM deposits were documented ().
FIGURE 1. Minimal change disease. Entirely normal appearing unremarkable glomeruli on background of nonspecific scanty scarring of the tubulointerstitium and single tubular atrophy. Moderate active inflammatory lymphocytic infiltrate with evidence of tubulitis and periglomerulitis (H&E, × 200).
The patient was initially treated with 75 mg/day prednisone and later on 60 mg/day with slow tapering. Four weeks later he presented no edemas with improvement in laboratory tests: hypoalbuminemia, 30 g/L; hypoproteinemia, 55 g/L; hypercholesterolemia, 7 mmol/L; hypertriglyceridemia, 3.5 mmol/L. Urine analysis showed slight proteinuria, blood counts returned to normal range but liver enzymes remained elevated (AST 121 IU/L, ALT 50 IU/L, GGT 2024 IU/L). The patient remained on low-dose prednisone therapy.
Three months later the patient developed an ischemic brain stroke with hemiparesis. Chronic GvHD was diagnosed, which progressed to extensive stage with skin and pulmonary manifestation (bronchiolitis). Laboratory assays revealed no hypoproteinemia with slight hypoalbuminemia. There was no relapse of nephrotic syndrome. The patient died 3 years later as a result of pulmonary infection, in a state of hematological complete remission.
CASE 2
A 30-year-old male (DT) with acute lymphoblastic leukemia in first RC (remission) underwent alloHSCT from matched family donor (his brother) 6 months from diagnosis. During remission induction, he was treated according to the Polish Adult Leukemia Group (PALG) protocol (DNR, VCR, CPD, Ara-C). In the consolidating therapy, he received CPD, Ara-C, MTX, and VePesid with no maintenance therapy as well as CNS prophylactic radiotherapy. Transplantation conditioning consisted of busulphan (16 mg/kg) and cyclophosphamide (120 mg/kg).
Seven months post-transplant a relapse was diagnosed and he underwent a second alloHSCT from his brother with the same conditioning chemotherapy. Three months post-transplant he developed chronic GvHD involving skin and mucous membranes. At that time he also developed proteinuria. He was treated with corticosteroids and cyclosporin A.
After 8 months, he was admitted to hospital due to severe nephrotic syndrome and induced generalized edema. Proteinuria amounted up to 6.7 g/day. Laboratory assays showed severe hypoalbuminemia (15 g/L), hypoproteinemia (34 g/L), hypercholesterolemia (19.5 mmol/L), hypertriglyceridemia (5.26 mmol/L), abnormal liver tests (GOT 264 IU/L, GPT 99 IU/L, GGTP 946 IU/L), and accelerated sedimentation rate (125 mm/h).
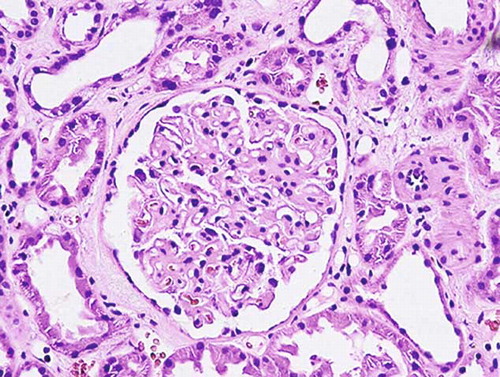
USG showed hyperechogenic kidneys with no structural abnormalities. Percutaneous kidney biopsy was performed revealing signs of membranous glomerulopathy. Renal histology showed discrete periglomerular fibrosis and no leukocyte infiltration in tubulointerstitium. Immunohistochemical analysis uncovered diffuse granular deposits of C3, IgM, IgA, and IgG in glomerular basement membrane ().
FIGURE 2. Membranous glomerulopathy. Evident generalized diffuse thickening of the capillary walls due to deposits within glomerular basement membrane (H&E, × 400).
The initial therapy included corticosteroids 60 mg/day and cyclosporine dosage was increased to 150 mg/day. The course was complicated by viral neuroinfection with tonic–clonic convulsions. Cyclosporin administration was halted and the patient was treated with dexamethasone, ganciclovir/acyclovir. In laboratory analysis, an improvement was seen in albumin levels (albumin serum concentration 21 g/L, total protein 55 g/L), lower concentration of cholesterol (10.6 mmol/L), and triglycerides (2.92 mmol/L) with no improvement of proteinuria which was 9.12 g/day. Liver tests remained elevated (AST 209 IU/L, ALT 43 IU/L, GGT 1519 IU/L). Therapy was continued with cyclosporin 150 mg/day and prednisone 50 mg/day.
After 3 months, mycophenolate mofetil was added due to persistence of chronic GvHD with cachexia, nephrotic syndrome, and infections (encephalitis). A month later acute lymphoblastic leukemia relapse was diagnosed with fatal outcome.
RT-PCR IN SITU
Expression of cytokine genes for tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), interferon gamma (IFN-γ), interleukin 2 (IL-2), IL-6, and IL-10 was assessed using semiquantitative evaluation with RT-PCR in situ on paraffin tissue sections from renal core needle biopsy. After RT-PCR amplification and fixation of reaction products, a mixture of digoxigenin-labeled specific sense and antisense oligonucleotide probes for in situ hybridization reactions were used (using starters obtained from R&D Systems). The sizes of the amplicons were TNF-α, 413 kb; TGF-β1, 442 kb; IFN-γ, 453 kb; IL-2, 357 kb; IL-6, 421 kb; IL-10, 427 kb, accordingly. After washing, slice specimens were incubated with anti-digoxigenin alkaline phosphatase conjugate and then with the chromogen mixture NBT/BCIP. mRNA transcripts were evaluated with the use of light microscopy and seen as granular precipitates which were quantified by calculating the mean number of positive points per area unit in all glomeruli and 20 hpf in the tubulointerstitium and pronounced as expression score. Results were corrected by β-actin gene expression using
forward primer: GATGACCCAGATCATGTTTG,
reverse primer: CTCCATGCCCAGGAAGGAAG,
antisense probe: Dig-GTGAGGATCTTCATGAG GTAGTCAGTCAGG-Dig,
sense probe: Dig-CATGTACGTTGCTATCCAG GCTGTGCTATC-Dig,
size of amplicon: 457 kb.
A normal kidney tissue specimen obtained from a living related donor during organ procurement served as a control.
In the two presented cases of GvHD-linked nephrotic syndrome, increased cytokine gene expression in comparison with normal kidney tissue was observed. The expression scores for TNF-α, TGF-β, IFN-γ, IL-2, IL-6, and IL-10 (in glomeruli as well as in tubulointerstitium) were five to nine times greater than in normal kidneys.
DISCUSSION
Kidney involvement in chronic GvHD is a rare complication and only a few dozen cases were described in publications.Citation2,Citation4–19 In a paper by Reddy et al., it has been reported that in a series of 889 patients undergoing alloHSCT, the incidence of nephrotic syndrome was 1%.Citation4 In recent analyses, the cumulative incidence of nephrotic syndrome ranged from 6.5 to 8%.Citation17,Citation18 Recipients grafted with peripheral blood stem cells had a higher probability of developing nephrotic syndrome than those grafted with bone marrow.Citation17 In all these reports, the onset of severe proteinuria occurred in 3–48 months after alloHSCT and usually was not preceded by acute GvHD.Citation20 The majority of patients presented other clinical manifestations of chronic GvHD before nephrotic syndrome onset.Citation4 Histological examination revealed membranous glomerulopathy (MG) in 70% and minimal lesions in 20% of cases. Two cases of diffuse proliferative glomerulonephritis with cellular crescents in patients after alloHSCT were also reportedCitation11,Citation12 as well as amyloidosisCitation21 or recipient residual lymphoma infiltrates.Citation22 The transfer of alloreactive donor lymphocytes reactive against human leukocyte antigens (HLAs) or glomerular basement membrane (GBM) antigens, expressed on the glomerular structures, may lead to in situ immune complex formation.Citation20 Some of the onsets of nephrotic syndrome were connected with cyclosporin therapy tapering or cessation but some of them occurred during cyclosporin administration.
The management of nephrotic syndrome due to GvHD usually bases on prednisone and cyclosporin administration, in rare cases on chlorambucil, azathioprine, mycophenolate mofetil, or rituximab usage. Immunosuppressive treatment usually reduces the symptoms of nephrotic syndrome; however, less than 50% of patients reach complete remission.Citation20 In a few cases the onset of nephrotic syndrome preceded (by 1–2 months) the relapse of extramedullary leukemia.Citation20
The pathological mechanisms of chronic GvHD still remain poorly elucidated. It is suggested that altered Th2 alloreactivity contributes to the development of chronic GvHD in humans.Citation23 Enhanced expression of Th2-derived cytokines was found in chronic GvHD suggesting similarity with autoimmune disorders due to dysregulation of the immune system. Romagnani has demonstrated that infiltrating mononuclear cells definitely originate from the transplant donor and not leukemia relapse.Citation16 Alloreactive T lymphocytes recognizing host minor HLA or non-HLA antigens lead to lymphocyte activation and autoantibody synthesis. Autoantibody formation has been documented in animal models as well in humans. An excellent response of patients to B-cell depletion (by anti-CD20) supports the hypothesis concerning B-cell importance in the development of chronic GvHD.Citation4 The immune antigen–autoantibody complexes may be deposited in various tissues, for example, kidney. MG associated with chronic GvHD may have similar pathogenesis as de novo occurrence of MG in transplanted kidneys. Altered host alloreactivity to major or minor HLA expressed in glomerular structures is postulated to be associated with post-transplant de novo MG which involves only the transplanted organ but not native kidneys.Citation9
Recently, however, the role of Th1 cells has been emphasized. In a recent paper by Seconi et al., an increase of TNF-α and IFN-γ expression by engrafted donor T cells accompanying the onset of nephrotic syndrome has been reported.Citation13 According to our knowledge, the above is a first-time report of increased cytokine gene expression in renal tissue during nephrotic syndrome due to chronic GvHD. Surprisingly, we have demonstrated not only increased Th2-derived cytokine gene expression (IL-10) but also Th1-derived (IFN-γ, IL-2) as well as monocyte/macrophage-derived (TNF-α, TGF-β, IL-6) expression. We are conscious of the limitations of the visualizing technique which we used (RT-PCR in situ). It did not allow for the determination of the cellular source of cytokine transcripts; therefore we cannot discriminate between cells infiltrating the tissue and the local cell population of the kidney. In this study we have also examined cytokine gene expression in the normal kidney tissue taken during living donation. The expression of all the observed mRNA in normal kidney tissue obtained from living donors was very low; however, none of the cytokine mRNA was absent.
In both cases studied we have observed significantly increased cytokine gene expression. In the first case (MCD) large lymphocyte infiltrates were present consisting mainly of T-lymphocytes which could be the main source of cytokines. In the second case (membranous GN) no infiltrates in the interstitium were found suggesting that the enhanced cytokine gene expression was derived from activated resident kidney cells as endothelial cells, podocytes, mesangial cells, and tubular epithelial cells. This also implies that (as demonstrated in animal models) tissue damage may result from T-cell recognition of alloantigens present at extrarenal sites,Citation13 and expression of cytokines are associated with organ damage.Citation24
In conclusion, this case report serves to emphasize the correlation between glomerulopathy and GvHD, also underscoring the fact that kidneys may be a target organ for chronic GvHD after alloHSCT.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lee S, Vogelsang G, Flowers M. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233.
- Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: Clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–129.
- Filipovich AH, Weisdorf D, Pavletic S, National Institutes of Health Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–955.
- Reddy P, Johnson K, Uberti JP, Nephrotic syndrome associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:351–357.
- Muller GA, Muller CA, Markovic-Lipkowski J, Membranous nephropathy after bone marrow transplantation in ciclosporin treatment. Nephron. 1989;51:555–556.
- Nergizoglu G, Keven K, Ates K, Chronic graft-versus-host disease complicated by membranous glomerulonephritis. Nephrol Dial Transplant. 1999;14:2461–2463.
- Barbara JA, Thomas AC, Smith PS, Gillis D, Ho JO, Woodroffe AJ. Membranous nephropathy with graft-versus-host disease in a bone marrow transplant recipient. Clin Nephrol. 1992;37:115–118.
- Yorioka N, Taniguchi Y, Shimote K, Membranous nephropathy with chronic graft-versus-host disease in a bone marrow transplant recipient. Nephron. 1998;80:371–372.
- Lin J, Markowitz GS, Nicolaides M, Membranous glomerulopathy associated with graft-versus-host disease following allogeneic stem cell transplantation. Report of 2 cases and review of the literature. Am J Nephrol. 2001;21:351–356.
- Akar H, Keven K, Celebi H, Nephrotic syndrome after allogeneic peripheral blood stem cell transplantation. J Nephrol. 2002;15:79–82.
- Suehiro T, Masutani K, Yokoyama M, Diffuse proliferative glomerulonephritis after bone marrow transplantation. Clin Nephrol. 2002;58:231–237.
- Kimura S, Horie A, Hiki Y, Nephrotic syndrome with crescent formation and massive IgA deposition following allogeneic bone marrow transplantation for natural killer cell leukemia/lymphoma. Blood. 2003;101:4219–4221.
- Seconi J, Watt V, Ritchie DS. Nephrotic syndrome following allogeneic stem cell transplantation associated with increased production of TNF-alpha and interferon-gamma by donor T cells. Bone Marrow Transplant. 2003;32:447–450.
- Oliveira JS, Bahia D, Franco M, Nephrotic syndrome as a clinical manifestation of graft-versus-host disease (GVHD) in a marrow transplant recipient after cyclosporine withdrawal. Bone Marrow Transplant. 1999;23:99–101.
- Sato N, Kishi K, Yagisawa K, Nephrotic syndrome in a bone marrow transplant recipient with chronic graft-versus-host disease. Bone Marrow Transplant. 1995;16:303–305.
- Romagnani P, Lazzeri E, Mazzinghi B, Nephrotic syndrome and renal failure after allogeneic stem cell transplantation: Novel molecular diagnostic tools for a challenging differential diagnosis. Am J Kidney Dis. 2005;46:550–556.
- Colombo AA, Rusconi C, Esposito C, Nephrotic syndrome after allogeneic hematopoietic stem cell transplantation as a late complication of chronic graft-versus-host disease. Transplantation. 2006;81:1087–1092.
- Srinivasan R, Balow JE, Sabnis S, Nephrotic syndrome: An under-recognised immune-mediated complication of non-myeloablative allogeneic hematopoietic cell transplantation. Br J Haematol. 2005;131:74–79.
- Brukamp K, Doyle A, Bloom R, Nephrotic syndrome after hematopoietic cell transplantation: Do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol. 2006;1:685–694.
- Stevenson WS, Nankivell BJ, Hertzberg MS. Nephrotic syndrome after stem cell transplantation. Clin Transplant. 2005;19:141–144.
- Ben-Dov I, Pizov G, Ben-Chetrit E. Fatal nephrotic syndrome complicating allogeneic stem cell transplantation: A case report. Nephrol Dial Transplant. 2009;24:2946–2949.
- Sakai K, Usui J, Kai H, Secondary membranous glomerulonephritis associated with recipient residual lymphoma cells after allogeneic bone marrow transplantation. Clin Exp Nephrol. 2009;13:174–178.
- Nakashima H. Membranous nephropathy is developed under Th2 environment in chronic graft-versus-host disease. Med Hypotheses. 2007;69:787–791.
- Lange A, Klimczak A, Karabon L, Cytokines, adhesion molecules (E-selectin and VCAM-1) and graft-versus-host disease. Arch Immunol Ther Exp. 1995;43:99–105.
