Abstract
Hepatitis B virus (HBV) infection is an uncommon cause of cryoglobulinemia. Renal cryoglobulinemia has been rarely reported in the setting of chronic hepatitis B infection. We describe a case of chronic hepatitis B infection with cryoglobulinemic glomerulonephritis (Gn) and provide information about the treatment and the evolution over a 30-month follow-up. A 41-year-old woman with chronic hepatitis B infection developed nephrotic syndrome and acute renal failure; other investigations revealed type 2 cryoglobulinemia; HBV DNA was detected in the cryoprecipitate. Renal biopsy showed findings of cryoglobulinemic Gn. She was given lamivudine, corticosteroids, plasma exchange, and mycophenolate mofetil. The renal function improved, nephrotic syndrome remitted, and HBV DNA became undetectable; there was no compromise of the liver function.
INTRODUCTION
Glomerulonephritis (Gn) is one of the extrahepatic complications associated with the hepatitis B virus (HBV). The relationship between the hepatitis C virus (HCV) and mixed cryoglobulinemia (MC) has been well established; however, the relationship between MC and HBV is more controversial. Cryoglobulins can be detected in the serum of patients with Gn associated with HBV,Citation1 but cases of cryoglobulinemic Gn and HBVCitation2,Citation3 are uncommon, and there is very little information about its evolution and treatment.
We describe the case of a patient with chronic HBV infection who developed acute renal failure and nephrotic syndrome secondary to cryoglobulinemic Gn.
CASE REPORT
A 41-year-old woman was referred to our department for impaired renal function and edema. Three and a half years earlier, she presented acute HBV infection; afterward the hepatitis B e antigen (HBeAg) became negative and she remained with the following serology: hepatitis B surface antigen (HBsAg)-positive, antihepatitis B core IgM antibody (anti-HBcIgM)-negative, anti-HBcIgG-positive, HBeAg-negative, anti-HBe-positive, and anti-HBs-positive; the transaminases were slightly elevated (AST (aspartate aminotransferase) 48 IU/L, ALT (alanine aminotransferase) 43 IU/L); and the creatinine was normal. She refused to have a liver biopsy.
One month before the current admission, she began experiencing edema in the lower limbs, dark urine, and progressive dyspnea; in the last week paresthesia appeared in the left lower limb. Physical examination revealed the following: afebrile, BP 170/90 mmHg; cardiac auscultation was rhythmic at 106 bpm; ascites and edema up to the knees; and the remainder within normal limits. The laboratory tests revealed normal coagulation, hemoglobin 10.2 g/dL, platelets 159,000/mm3, urea 138 mg/dL, creatinine 2.17 mg/dL, albumin 3.3 g/dL, potassium 5.5 mEq/L, CRP 55 mg/L (nv < 2), AST 33 IU/L (nv 10–35), ALT 46 (nv 10–34) IU/L, IgG 544 mg/dL, IgA 269 mg/dL, IgM 490 mg/dL, rheumatoid factor 830.59 IU/mL (nv < 14), C3 66 mg/dL (nv>93), and C4 < 1 mg/dL (nv>15); high-resolution electrophoresis and immunofixation of serum revealed a monoclonal IgM-kappa component, the cryoglobulins, characterized as monoclonal IgM kappa-polyclonal IgG, were positive. The rest of the elemental biochemistry, antistreptolysin O (ASTO), antinuclear antibodies (ANA), antideoxyribonucleic acid antibodies (anti-DNA), antineutrophil cytoplasmic antibodies (ANCA), and antiglomerular basement menbrane antibodies (anti-GBM) were all normal/negative. Urine: proteinuria was 6.5 g/24 h (high-resolution electrophoresis/immunofixation: monoclonal kappa light chains 272 mg/L); urinary sediment: uncountable RBC/HPF (70% dysmorphic), negative urine culture. Viral serology: hepatitis A virus (HAV) IgM-negative, anti-hepatitis delta virus (HDV)-negative, anti-HCV-negative, HIV-negative, reverse transcriptase-polymerase chain reaction (RT-PCR) HBV (COBAS ROCHE, Branchburg, NJ, USA) 630,000 copies/mL, HBV genotype A, PCR HCV (COBAS ROCHE, Branchburg, NJ, USA)-negative, RT-PCR HBV in cryoglobulins 125,000 copies/mL, PCR VHC in cryoglobulins negative; the rest of HBV serology was as above and that was compatible with an HBV precore mutant infection. Chest X-ray: bilateral pleural effusion; ECG and echocardiography: normal. Abdominal ultrasonography revealed normal kidneys and liver. The electromyography disclosed reduced amplitude in sensory potential of the left peroneal nerve.
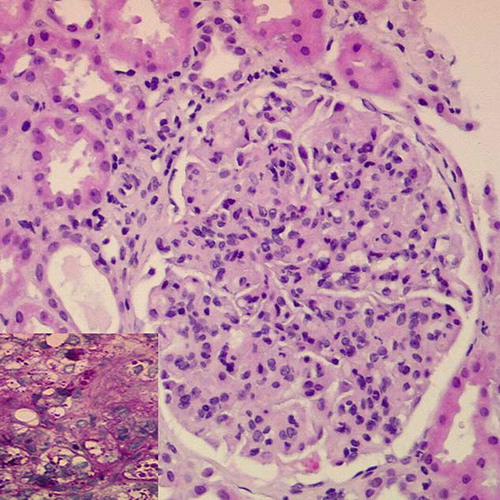
The patient was diagnosed as having chronic HBV infection, type 2 MC and probable proliferative Gn. The creatinine worsened to 3.4 mg/dL; lamivudine 50 mg/day was administered as well as three bolus of methylprednisolone (0.5 g each). Percutaneous renal biopsy (15 glomeruli) showed diffuse endocapillary proliferation, thickening and double contour appearance of the glomerular basement membrane, and leukocytes infiltration; focally, the capillary lumen showed PAS+ hyaline thrombi (); the interstitium contained patchy mononuclear cell infiltration. The immunofluorescence revealed granular deposits of IgM (3+), IgG (2+), C3 (3+), C1q (1+), kappa (2+), and lambda (1+) on capillary walls; electron microscopy of a deparaffinized sample disclosed hyaline thrombi and basement membrane reduplication. These findings were compatible with renal involvement in MC. A fresh sample of the renal biopsy was studied with nested-PCR and was positive for HBV DNA and negative for HCV RNA.
FIGURE 1. Renal biopsy (HE, 20× magnification). Glomerulus showing endocapillary proliferation, thickening of the capillary walls, and leukocytes infiltration. Inset: glomerular detail (PAS, 40× magnification) demonstrating intracapillary thrombi.
The patient continued treatment with lamivudine and oral prednisone (1 mg/kg/day for 8 weeks and then a progressive tapering); six plasma exchanges were performed (3 L each) with the addition of mycophenolate mofetil 500 mg/8 h. The creatinine returned to normal (0.95 mg/dL) 1 month after starting treatment; the proteinuria dropped to below 0.5 g/24 h at 4 months; HBV DNA levels became undetectable at 10 months. The prednisone was maintained for 7 months; mycophenolate mofetil for 10 months, and the patient continued with lamivudine 100 mg/day. At 30 months after diagnosis, the findings were as follows: creatinine 0.85 mg/dL, proteinuria 103 mg/24 h, normal sediment, normal transaminases, PCR HBV undetectable, cryoglobulins negative, C3 normal, C4 < 1 mg/dL, and rheumatoid factor 68.69 IU/mL. The bone marrow aspiration, electromyography, and chest-abdominal-pelvic CT-scan showed no abnormalities. A summary of the evolution is presented in .
TABLE 1. Summary of the evolution
DISCUSSION
The main histological patterns of Gn associated with HBV are membranoproliferative, membranous, and IgA nephropathy.Citation4 The basic pathogenic mechanism of HBV-Gn would be the deposit of immune complexes (HBV-antibodies), although the cytopathic effect of the virus may also intervene together with T-cell- and cytokine-mediated damage.Citation5
One particular type of immune complex would be that made up of cryoglobulins. The relationship between HBV and cryoglobulinemia is controversial. Levo et al.Citation6 suggested that they were associated, but it was not demonstrated in later studies.Citation7,Citation8 It is now considered that the HBV can produce cryoglobulinemia but only rather infrequently.Citation9 For example, in a study of 190 patients with chronic HBV infection, only three had positive cryoglobulins.Citation10 In another study of 231 patients with MC, the HBsAg was positive in 9% and HBV DNA in 1.8%.Citation11 In our patient, the pathogenic role of HBV in cryoglobulinemia was confirmed by the positive finding for the HBV DNA in the cryoprecipitate, whereas the HCV RNA was negative.
HBV may produce cryoglobulins in a similar way to HCV.Citation12 The antigenic stimulus maintained by HBV would determine the polyclonal and later oligomonoclonal expansion of B-cells with the appearance of cryoglobulins and rheumatoid factor. Immune complexes would be formed between HBV, polyclonal IgG, and monoclonal IgM and deposited on a tissue level. The monoclonal IgM component with rheumatoid factor activity is considered to be key in kidney deposits.
In this case, the renal biopsy showed type 1 membranoproliferative Gn with hyaline thrombi, findings that were compatible with cryoglobulinemic Gn. The most common glomerular histological pattern in monoclonal MC is type 1 membranoproliferative GnCitation13; the presence of hyaline thrombi is more inconsistent and has been related to the development of an acute nephritic syndrome,Citation14 as in the case of this patient. Electron microscopy did not reveal any crystalline structures typical of cryoglobulinemia, but these structures are not always observed.
The best treatment for cryoglobulinemic Gn associated with HBV is not well established. Whenever HCV-related Gn is associated with nephrotic syndrome and/or rapidly renal insufficiency, it has been suggested to use immunosuppressive therapy (corticosteroids, cyclophosphamide, rituximab) and plasma exchange, and, in a second phase, antiviral treatment.Citation15 We use antiviral therapy in combination with immunosuppressors. Lamivudine is a nucleoside analogue that inhibits HBV DNA polymerase and has been proven effective in HBV-Gn with nephrotic syndrome and impaired renal function.Citation16,Citation17 Lamivudine inhibits the replication of HBV and so reduces the antigenic stimulus on the B-cells and the production of cryoglobulins. Lamivudine is fast acting and not immunomodulating like interferon. The drawback of lamivudine is that it must be administered over a long period, especially in HBeAg-negative patients, and it may cause the appearance of HBV-resistant forms. However, some authors have administered lamivudine for years without complications.Citation18,Citation19 Mycophenolate mofetil has been used in HCV-associated cryoglobulinemia with good resultsCitation20 and seems to be a drug with fewer side effects than cyclophosphamide and rituximab. Its role in HCV/HBV-associated cryoglobulinemia therapy is not defined but could be an alternative option. Progress has been favorable in our patient during the 30-month follow-up; there have been no treatment-related complications, HBV DNA is undetectable, and the liver function has not deteriorated. Nevertheless, the cryoglobulinemia has not been cured, merely controlled; the positivity of the rheumatoid factor and low C4 would indicate cryoglobulinemic activity below the limit of detection.
In summary, cryoglobulinemic Gn is an uncommon complication of chronic HBV infection as shown in this report that provides information about its treatment and evolution.
Acknowledgments
We thank prof. J. Forteza (Pathology Department, Hospital Clínico Universitario, Santiago de Compostela) for his assistance with electron microscopy study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Venkataseshan VS, Lieberman K, Kim DU, Hepatitis-B-associated glomerulonephritis: Pathology, pathogenesis, and clinical course. Medicine (Baltimore). 1990;69:200–216.
- Perez GO, Pardo V, Fletcher MA. Renal involvement in essential mixed cryoglobulinemia. Am J Kidney Dis. 1987;10:276–280.
- Bouhsain S, Ouzzedoun N, Tellal S, Vascularite rénale dans le cadre d`une cryoglobulinémie mixte associée au virus de l´hépatite B. Ann Biol Clin. 2007;65:643–646.
- Di Belgiojoso GB, Ferrario F, Landriani N. Virus-related glomerular diseases: Histological and clinical aspects. J Nephrol. 2002;15:469–479.
- Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol. 2004;24:198–211.
- Levo Y, Gorevic PD, Kassab HJ, Zucker-Franklin D, Frankin EC. Association between hepatitis B virus and essential mixed cryoglobulinemia. N Engl J Med. 1977;296: 1501–1504.
- Fiorini G, Bernasconi P, Sinico RA, Chianese R, Pozzi F, D´ Amico G. Increased frequency of antibodies to ubiquitous viruses in essential mixed cryoglobulinemia. Clin Exp Immunol. 1986;64:65–70.
- Galli M. Cryoglobulinaemia and serological markers of hepatitis viruses. Lancet. 1991;338:758–759.
- Trejo O, Ramos-Casals M, García-Carrasco M, Cryoglobulinemia. Study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001;80:252–262.
- Cacoub P, Saadoun D, Bourlière M, Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43:764–770.
- Ferri C, Sebastiani M, Giuggioli D, Mixed cryoglobulinemia: Demographic, clinical and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355–374.
- Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25.
- Beddhu S, Bastacky S, Johnson JP. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine (Baltimore). 2002;81:398–409.
- D´Amico G. Renal involvement in hepatitis C infection: Cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671.
- Fabrizi F, Lunghi G, Messa P, Martin P. Therapy of hepatitis C virus-associated glomerulonephritis: Current approaches. J Nephrol. 2008;21:813–825.
- Wen YK, Chen ML. Remission of hepatitis B virus-associated membrano- proliferative glomerulonephritis in a cirrhotic patient after lamivudine therapy. Clin Nephrol. 2006;65:211–215.
- Di Marco V, De Lisi S, Vecchi ML, Maringhini S, Barbaria F. Therapy with lamivudine and steroids in a patient with acute hepatitis B and rapidly progressive glomerulonephritis. Kidney Int. 2006;70:1187–1188.
- Izzedine H, Massard J, Poynard T, Deray G. Lamivudine and HBV-associated nephropathy. Nephrol Dial Transplant. 2006;21:828–829.
- Mesquita M, Lasser L, Langlet P. Long-term (7-year-) treatment with lamivudine monotherapy in HBV-associated glomerulonephritis. Clin Nephrol. 2008;70:69–71.
- Reed MJ, Alexander GJM, Thiru S, Smith KGC. Hepatitis C-associated glomerulonephritis – A novel therapeutic approach. Nephrol Dial Transplant. 2001;16:869–871.
