Abstract
A 10-year-old girl was admitted with fever, cough, maculopapular rash, hemoptysis, dark-colored urine, edema, multiple lymphadenopathies, and hepatosplenomegaly. She developed acute glomerulonephritis during the course of these complex clinical features. Laboratory data showed hematuria, proteinuria, and hypocomplementemia. Serological tests showed positive human parvovirus B19 (HPVB19)-specific immunoglobin M (IgM) and HPVB19 DNA was detected in the patient's serum using polymerase chain reaction (PCR). Renal biopsy revealed acute endocapillary proliferative glomerulonephritis (AEPGN) with coarse granular C3 depositions in a “starry sky pattern,” which is more peculiar to poststreptococcal glomerulonephritis. Electron microscopy showed subendothelial and small hump-shaped subepithelial electron-dense deposits in glomerular capillary walls. There was no evidence of either any mycobacterial, staphylococcal, or streptococcal infection, and the diagnosis of Goodpasture syndrome and connective tissue disorders was excluded during clinical and laboratory investigations. A diagnosis of HPVB19-induced pleuropneumonitis and glomerulonephritis was made. Through a literature search there was no documented pediatric case of AEPGN induced by HPVB19, and this case represents, to our knowledge, the first time that a direct relationship between parvovirus infection and AEPGN has been demonstrated in a child.
INTRODUCTION
Human parvovirus B19 (HPVB19) was identified as the cause of a self-limited childhood febrile illness with rash, namely, erythema infectiosum or fifth disease.Citation1 Other clinical manifestations that can occur with HPVB19 infection include arthritis or arthralgia, transient aplastic crisis, fetal hydrops, and chronic infection with anemia.Citation2 Most of the HPVB19 infections are usually mild or asymptomatic, and multisystem involvement simulating a connective tissue disorder or malignancy is extremely rare. However, in some cases, infection is associated with serious systemic complications for which treatment is indicated and may be life saving.Citation2,Citation3 Renal involvement in patients with HPVB19 infection was not discussed in early reports, but a causal relationship between this infection and acute glomerulonephritis has been suggested in recent, mostly anecdotal, case reports.Citation1–16 The majority of these reports were described in adults, whereas only a few cases of children were defined who presented with mesangiocapillary proliferative glomerulonephritis, focal segmental glomerulosclerosis (FSGS), or tubulointerstitial nephritis. We present the first pediatric case of acute endocapillary proliferative glomerulonephritis (AEPGN) induced by HPVB19 that did not resolve spontaneously.
CASE REPORT
A 10-year-old girl was admitted with symptoms of rash, fever, cough, hemoptysis, dark-colored urine, and edema. Her medical history revealed Raynaud's phenomenon for 3 years and an episode of pneumonia within 2 weeks. Her family history was unremarkable but her parents were cousins. Her body temperature was 38.8˚C and blood pressure was 110/60 mmHg. Physical examination revealed periorbital and pretibial edema, cervical, submandibular, axillary, and inguinal multiple lymphadenopathies, hepatosplenomegaly, and generalized itchy erythematous maculopapular rash. Decreased breath sounds over the right lung and secretion rales were present on auscultation. Systemic examination was otherwise unremarkable.
Laboratory results on admission showed a hemoglobin of 10.5 g/dL, hematocrit 31.5%, white blood cell 6800/mm3, platelets 233,000/mm3, erythrocyte sedimentation rate (ESR) 87 mm/h (0–20), C-reactive protein (CRP) 92 mg/L (0–10), and fibrinogen 522 mg/dL (200–400). Urinalysis revealed proteinuria (11 mg/m2/h) and hematuria with dysmorphic erythrocytes and red blood cell casts. Creatinine clearance was 110 mL/min/1.73 m2. Serum blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, glucose, and electrolytes were normal. Blood cultures were sterile on three occasions, whereas the urine cultures and throat cultures were sterile on two. Chest X-ray examination showed right-sided hilar adenopathy and collapse-consolidation lesions. Ultrasound examination revealed bilateral pleural effusion. Pleural fluid was hemorrhagic but did not show any malignant cells, and cultures were negative on repeated occasions. Computed tomography of thorax revealed mediastinal and hilar adenopathies accompanied with bilateral consolidation and atelectasia of lower lobes. Bronchoscopy demonstrated bronchiectasis of the right middle lobe segments and basal segments of the lower lobes. Neutrophil leukocytes and alveolar macrophages were observed on cytologic examination of endobronchial secretions and cultures were negative. Tuberculin skin test was nonreactive. Three consecutive morning gastric aspirates yielded no organisms, and acid-fast smear of sputum, endobronchial secretions, and urine was negative. Cultures for Mycobacterium tuberculosis were negative and also the presence of M. tuberculosis in these specimens could not be detected by using polymerase chain reaction (PCR) assay.
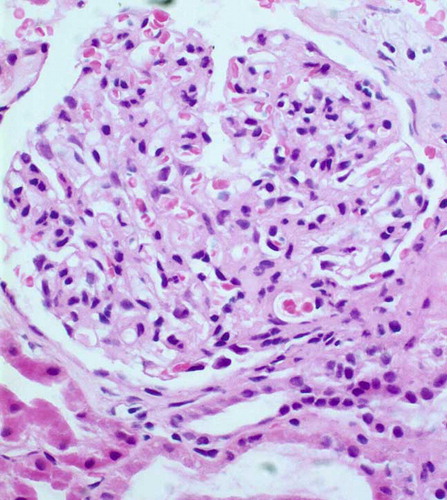
A provisional diagnosis of disseminated staphylococcal or streptococcal disease with septicemia was made, and she was given benzathine penicillin and continued with vancomycin and ceftriaxone treatment for 2 weeks, but she continued to have fever and macroscopic hematuria. The only improvement was some relief of the respiratory distress and hemoptysis. Antimicrobials were omitted, as the possibility of drug-induced fever could not be negated. At this point, the course of the illness helped us to recognize the multisystemic nature of this disease, which prompted us to consider a vasculitic process such as systemic lupus erythematosus (SLE), polyarteritis nodosa (PAN), or Goodpasture's syndrome. Reports of investigations directed toward these etiologies revealed negative rheumatoid factor, anti nuclear antibody (ANA), anti-dsDNA, antineutrophil cytoplasmic antibodies, antiglomerular basal membrane (anti-GBM) antibodies, and extractable nuclear antigens (ENA) test. Serologic work-up showed hypocomplementemia with a C3 concentration of 46 mg/dL (80–150) and C4 of 10 mg/dL (20–40). Antistreptolysin O titers (ASO), serum IgA, and IgM were within the normal range, but IgG and IgE levels showed an elevated pattern. Flow cytometry of lymphocyte subsets was normal. Cold agglutinins and cryoglobulin were negative. Serum angiotensin-converting enzyme level and sweat testing were also normal. Virologic studies showed a significant titer of HPVB19-specific IgM antibody and serum HPVB19 DNA was also detected using PCR. Hepatitis B and C, cytomegalovirus, Epstein–Barr virus, herpes simplex types I and II, measles, rubella, varicella, mumps, and coxsackie virus infections were negated by the absence of serologic responses. Abdominal ultrasonography demonstrated hepatosplenomegaly and bilateral enlarged kidneys. There was no evidence of M. tuberculosis infection on intravenous pyelographic investigation. Renal biopsy was performed 3 days after admission. Light microscopy showed mild endocapillary proliferation with glomerular leukocyte infiltration and endothelial cell swelling. There was a mild increase of segmental mesangial matrix (). The interstitium was slightly edematous, and mild tubulus degeneration was observed. By immunofluorescent study, coarse granular C3 depositions were found in the mesangium and along the GBM (starry sky pattern). On electron microscopy, numerous subendothelial electron-dense deposits were observed and small hump-shaped subepithelial deposits were present in some glomerular capillary walls.
A final diagnosis of HPVB19-induced pleuropneumonitis and glomerulonephritis was made. After hospitalization with bed rest for 3 weeks, the patient recovered spontaneously. Except microscopic hematuria, other clinical symptoms such as hemoptysis, proteinuria, and macroscopic hematuria disappeared. Hypocomplementemia resolved and ESR and CRP also decreased. But on the 25th day of hospitalization macroscopic hematuria recurred. ESR and CRP increased again, whereas C3 level decreased, so she was given 2 mg/kg/day of prednisone. By week 6, her macroscopic hematuria resolved and C3, ESR, and CRP became normal. She was discharged 45 days after admission and steroid treatment at a dose of 1 mg/kg/day was maintained. She was asymptomatic at her recent follow-up examination. Serum HPVB19 DNA was not found, even through PCR analysis. In addition, the HPVB19-specific IgM seroconversion disappeared, whereas IgG seroconversion occurred and other laboratory values were within the normal range.
DISCUSSION
HPVB19 is a DNA virus and the only known parvovirus to infect humans. The infection can vary from an asymptomatic or subclinical infection to a serious biphasic illness, even in the normal host.Citation17 Multisystem disease in immunocompetent individuals is very rare, and there are only a few case reports in the literature.Citation3,Citation18
The complex clinical features of our patient including rash, fever, cough, hemoptysis, dark-colored urine, edema, multiple lymphadenopathies, hepatosplenomegaly, and pulmonary findings led us to make a differential diagnosis of acute/chronic infectious diseases such as disseminated staphylococcal/streptococcal/mycobacterial disease or any of vasculitic processes. The considered etiologies were adequately ruled out during laboratory inquires. Through an excessive investigation, the combination of peripneumonitis, hematuria, proteinuria, and transient reduction in plasma complement with an increase of acute phase reactants, together with the serological findings and typical renal histological findings in this patient, led to a diagnosis of multisystemic vasculitis and AEPGN associated with HPVB19 infection. Many reports have suggested that a combination of serum HPVB19 DNA and HPVB19 IgM could provide higher diagnostic sensitivity for acute disease.Citation19–22 It would be better if we could have demonstrated the presence of HPVB19 virus in renal biopsy specimen by immunochemistry or in situ hybridization, but it was impossible because of some technical problems.
Recently, HPVB19 infection has been increasingly described in association with various renal diseases. Wierenga et al. first defined seven patients (including three children) with homozygous sickle cell disease presenting with nephrotic syndrome induced by HPVB19 infection.Citation4 Chakravarty and Merry reported that an adult patient without hematological disease had moderate proteinuria with parvovirus infection that showed mild mesangioproliferative glomerulonephritis on histopathologic investigation.Citation5 A pediatric case of FSGS with sickle cell disease was reported by Tolaymat et al., in which they demonstrated HPVB19 DNA in renal specimen by PCR.Citation6 In another study the prevalence of HPVB19 DNA in renal biopsy specimens was found greater among patients with FSGS than other glomerular disease.Citation7 The specific association between HPVB19 infection and a distinct variant of FSGS called collapsing glomerulopathy was shown recently.Citation8 HPVB19 infection has also been associated with hemolytic uremic syndrome in adults without any underlying diseases.Citation9Citation,10 Murer et al. reported a relationship between HPVB19 infection and another form of thrombotic microangiopathy in four patients (including one adolescent) under immunosuppression after renal transplantation.Citation11 HPVB19-related central nervous system vasculitis and persistent anemia or pancytopenia has also been reported in renal transplant children.Citation23Citation,24 There have been reported pediatric cases of tubulointerstitial nephritisCitation12 and nephrotic syndrome,Citation13 which were associated with HPVB19 infection in the literature. Mori et al. reviewed the association of HPVB19 infection with acute glomerulonephritis in healthy adults and reported some common features, including female patient predominance in the second and third decades, hypocomplementemia, endocapillary or mesangiocapillary glomerulonephritis with subendothelial deposits, and spontaneous recovery.Citation2 On the contrary, only a small percentage of the patients discussed in the literature required immunosuppressive treatment.Citation7Citation,8,Citation14 A 17-year-old immunocompetent patient was reported with not-spontaneously resolving acute glomerulonephritis after HPVB19 infection whose renal biopsy was consistent with mild mesangial proliferation and focal segmental sclerosis.Citation14
Characteristics of our patient were in accordance with the literature, but the most striking feature during the hospital stay was the recurrence of the hematuria accompanied with hypocomplementemia, and corticosteroid treatment was thought to be necessary for multisystemic involvement of the disease. Lung involvement with HPVB19 is less frequent than renal disease but has been previously described in a few patients.Citation3,Citation25–27 As in our patient, only Dass et al. described a case of lung and renal involvement in the same patient.Citation3
We consider that our case represents another example of immune-complex-mediated AEPGN following HPVB19 infection, because of the presence of specific IgM and HPVB19 DNA in the sera detected using PCR in conjunction with the absence of any other detectable agents or processes. Iwafuchi et al. reported an adult case of AEPGN following HPVB19 infection whose histological findings were similar with poststreptococcal glomerulonephritis.Citation1 They stated that there were certain apparent differences between these two conditions such as the absence of large subepithelial electron-dense deposits (humps) and the different immunofluorescence pattern from three types (garland, starry sky, and mesangial patterns) of poststreptococcal glomerulonephritis. Although the histological findings of our patient were consistent with those of poststreptococcal glomerulonephritis, there was no evidence of any streptococcal infection. In contrast with the report by Iwafuchi et al.,Citation1 immunofluorescent study of our patient showed a “starry sky pattern” with coarse granular C3 depositions in the mesangial regions and along the glomerular capillary walls, and electron microscopy revealed numerous subendothelial and hump-shaped subepithelial electron-dense deposits. Although “starry sky pattern” is one of the most prototypic features of poststreptococcal glomerulonephritis, it can be accompanied with many postinfectious glomerulonephritis as in our patient.
In conclusion, as far as multisystem involvement is concerned, characteristics of our child resemble the other cases described previously. Based on the literature search, to our knowledge, the case presented here seems to be the first pediatric case of AEPGN induced by HPVB19 infection. In addition, different histological patterns and clinical courses can be seen following HPVB19 infection. While most of them resolve spontaneously, the necessity of corticosteroids should be taken into account in some cases.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Iwafuchi Y, Morita T, Kamimura A, Kunisada K, Ito K, Miyazaki S. Acute endocapillary proliferative glomerulonephritis associated with human parvovirus B19 infection. Clin Nephrol. 2002;57:246–250.
- Mori Y, Yamashita H, Umeda Y, Association of parvovirus B19 infection with acute glomerulonephritis in healthy adults: Case report and review of the literature. Clin Nephrol. 2002; 57:69–73.
- Dass R, Ramesh P, Ratho RK, Saxena AK, Singh S. Parvovirus B19-induced multisystem disease simulating systemic vasculitis in a young child. Rheumatol Int. 2005;25:125–129.
- Wierenga KJJ, Pattison JR, Brink N, Glomerulonephritis after human parvovirus infection in homozygous sickle-cell diseases. Lancet. 1995;346:475–476.
- Chakravarty K, Merry P. Systemic vasculitis and atypical infections: Report of two cases. Postgrad Med J. 1999;75: 544–546.
- Tolaymat A, Mousily FA, MacWilliam K, Lammert N, Freeman K. Parvovirus glomerulonephritis in a patient with sickle cell disease. Pediatr Nephrol. 1999;13:340–342.
- Tanawattanacharoen S, Falk RJ, Jennette JC, Kopp JB. Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis. 2000;35:1166–1174.
- Moudgil A, Nast CC, Bagga A, Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001;59:2126–2133.
- Seward EW, Rustom R, Nye FJ, Bone M. Hemolytic-uremic syndrome following human parvovirus infection in a previously fit adult. Nephrol Dial Transplant. 1999;14:2472–2473.
- Iwafuchi Y, Morita T, Kamimura A, Kunisada K, Kunisada K, Miyazaki S. Human parvovirus B19 infection associated with hemolytic uremic syndrome. Clin Exp Nephrol. 2000;4:156–159.
- Murer L, Zacchello G, Bianchi D, Thrombotic microangiopathy associated with parvovirus B19 infection after renal transplantation. J Am Soc Nephrol. 2000;11:1132–1137.
- Ramirez JA, Coccia PA, Ferrero HA, Livellara B, Christiansen S, Gallo G. Tubulointerstitial nephritis associated with parvovirus beta 19 infection. Medicina (B Aires). 2005;65:333–337.
- Ohtomo Y, Kawamura R, Kaneko K, Nephrotic syndrome associated with human parvovirus B19 infection. Pediatr Nephrol. 2003;18:280–282.
- Onguru P, Dede F, Bodur H, Glomerulonephritis associating parvovirus B19 infection. Ren Fail. 2006;28:85–88.
- Komatsuda A, Othani H, Nimura T, Endocapillary proliferative glomerulonephritis in a patient with parvovirus B19 infection. Am J Kidney Dis. 2000;36:851–854.
- Takeda S, Takaeda C, Takazakura E, Joji H. Renal involvement induced by human parvovirus B19 infection. Nephron. 2001;89:280–285.
- Brown KE. Parvovirus B19. In: Mandell GL, ed. Principles and Practice of Infectious Diseases. 5th ed., New York: Churchill Livingstone Inc.; 2000:1685–1691.
- Finkel TH, Torok TJ, Ferguson PJ, Chronic parvovirus B19 infection and systemic necrotizing vasculitis: Opportunistic infection or etiological agent. Lancet. 1994;343:1255–1258.
- Miron D, Luder A, Horovitz Y, Acute human parvovirus B-19 infection in hospitalized children: A serologic and molecular survey. Pediatr Infect Dis. 2006;25:898–901.
- Brown KE. Parvovirus B19. In: Mandell GL, Bennet JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed., New York: Churchill Livingstone Inc.; 2004:1891–1898.
- Anderson MJ, Higgins PG, Davis LR, Experimental parvoviral infection in humans. J Infect Dis. 1985;152:257–265.
- Patou G, Myint S, Pattison J. Characterization of a nested polymerase chain reaction assay for detection of parvovirus B19. J Clin Microbiol. 1993;31:540–546.
- Bilge I, Sadikoglu B, Emre S, Sirin A, Aydin K, Tatli B. Central nervous system vasculitis secondary to parvovirus B19 infection in a pediatric renal transplant patient. Pediatr Nephrol. 2005; 20:529–533.
- Laurenz M, Winkelmann B, Roigas J, Zimmering M, Querfeld U, Müller D. Severe parvovirus B19 encephalitis after renal transplantation. Pediatr Transplant. 2006;10:978–981.
- Bousvaros A, Sundel R, Thorne GM, Parvovirus B19 associated interstitial lung disease, hepatitis and myositis. Pediatr Pulmonol. 1998;26:365–369.
- Wardeh A, Marik P. Acute lung injury due to parvovirus pneumonia. J Intern Med. 1998;244:257–260.
- Morris CN, Smilack JD. Parvovirus B19 infection associated with respiratory distress. Clin Infect Dis. 1998;27:900–901.