Abstract
Obesity is currently an epidemic across the globe. Obese patients unable to achieve significant weight loss with lifestyle changes alone may require drug therapy. Clinical trials have shown that orlistat administration may not only lead to weight loss but also protect against type 2 diabetes in around 37% of cases. Orlistat can induce and maintain weight loss, even in patients with comorbid conditions such as hypertension or type 2 diabetes. Recently, orlistat can induce marked weight loss in individuals with chronic kidney disease (CKD). In small numbers of individuals especially those with CKD, orlistat administration may precipitate oxalate nephropathy and renal stone disease. The focus of this article is to review current studies showing impact of orlistat on renal function and outcomes.
INTRODUCTION
Overweight (defined as body mass index, BMI, of 25–30 kg/m2) and obesity (BMI > 30 kg/m2) have become serious threat to our health across the globe. The current obesity epidemic across the globe is associated with significant increase in morbidity and mortality. Importantly, there is an association between obesity and significant increase in renal diseases. Obesity is regarded as modifiable risk factor for renal diseases.Citation1 The features of the metabolic syndrome (insulin resistance, dysglycemia, dyslipidemia, hypertension, and central obesity) are not only risk factors for cardiovascular disease but also for renal disease.Citation2,Citation3 Importantly, obesity may be associated with end-stage renal disease (ESRD), diabetes, hypertension, and nephrotic syndrome.Citation4,Citation5 Interestingly, obesity-related glomerulopathy is associated with glomerulomegaly and focal segmental glomerulosclerosis.Citation6 Epidemiologic studies have shown that obesity is also associated with increased risk of renal stone formation. However, once BMI is greater than 30 kg/m2, further increases do not appear to significantly increase the risk of renal stone disease.Citation7 Medical treatment of obesity and lifestyle modification are two means in treating obesity in large proportions of obese individuals especially in primary health care.Citation2 Currently, bariatric surgery beside it is an expensive procedure, is regarded as last effective choice in maintaining significant weight loss in morbid obese individuals. Therefore, anti-obesity medications are widely prescribed before bariatric surgery.Citation8 Orlistat is widely used in Europe and North America for treatment of obesity.
ORLISTAT AND CALCIUM OXALATE CRYSTALLURIA
Orlistat (Xenical) inhibits up to 30% of ingested dietary fats and calories. Orlistat inhibits gastrointestinal production of gastric and pancreatic lipase (an enzyme that is essential for digestion of dietary triglycerides).Citation9 Orlistat, a synthetic derivative of lipstatin, also impairs digestion of dietary triglycerides and vitamin esters. It has no effect on systematic lipases as less than 1% of ingested orlistat is absorbed. The current recommended dose of orlistat is 120 mg, three times a day with meals.Citation9 Higher dose than this is unlikely to increase fat malabsorption. Data from a 4-year trial conducted in over 3000 obese subjects demonstrated that orlistat therapy and lifestyle intervention resulted in weight loss of 11% and 7% at 1 and 4 years, respectively, compared with 6% and 4% at 1 and 4 years for the group treated with placebo and lifestyle intervention. In addition, therapy with orlistat resulted in a 37% reduction in the cumulative incidence of new-onset type 2 diabetes, primarily by preventing the development of diabetes in patients who had impaired glucose tolerance.Citation10 Recently, orlistat is also shown to reduce weight in patients with CKD.Citation11 Administration of orlistat can be associated with gastrointestinal disorders (abdominal pain, flatulence, increased defecation, fecal urgency, and fatty/oily stool).
As consequence of dietary inhibition of fat absorption, possible side effect is increased risk of stone formation due hyperoxaluria.Citation12 The likely mechanism of hyperoxaluria with administration of gastrointestinal lipase inhibitors may be because of increased colonic hyperabsorption of oxalate. This may be because of presence of unabsorbed fat and bile acids in the lumen, which may react with calcium and ultimately increase enteric oxalate absorption and hyperoxaluria. Furthermore, the presence of free fatty acids in lumen have been shown not only to increase permeability for oxalate but also to increase concentration of soluble oxalate.Citation12,Citation13 Importantly, administration of orlistat is being shown to be associate not only with significant hyperoxaluria in human and animal models but also with significant increase in the risk of oxalate nephropathy. Ferraz et al. showed that in 39 male adult Wistar rats, the use of orlistat, especially under a diet rich in oxalate alone or associated with fat, leads to a significant and marked increase in urinary oxalate and a slight reduction in urinary calcium and urinary magnesium and ultimately increase the risk of urinary saturation of calcium oxalate. In their study, Ferraz et al. showed a reduction but not to significant level in urinary citrate (low urinary citrate is presumed as risk factor for stone formation). The authors concluded that orlistat may have the potential of increasing risk of stone formation.Citation14
Sarica et al. examined the possible effects of orlistat on the intestinal absorption of oxalate and thereby on the urinary levels of oxalate excretion in overweight 95 cases (57 men, 38 women; M/W = 1.5). Patients were randomly assigned into two groups. Whereas the patients in group I (n = 55) were treated with orlistat for 6 months, patients in group II (n = 40) received no specific medication. Calcium, oxalate, and citrate levels were determined in a 24-hour urine collection from each patient. Comparative evaluation of urinary oxalate levels during 3-month follow-up clearly showed that urinary oxalate excretion significantly increased in 34/55 patients (61.8%) in the first group (p < 0.05). Of these 34 patients, 30 (88.2%) continued to have increased urinary oxalate excretion during 6-month follow-up (p = 0.001). However, orlistat did not show any significant effect of this medication on urinary citrate and calcium levels during 3- and 6-month follow-up evaluation (p = 0.05). The authors again suggested that increased intestinal absorption of dietary oxalate due orlistat could make a substantial contribution to urinary oxalate excretion and may increase the risk of stone formation.Citation15
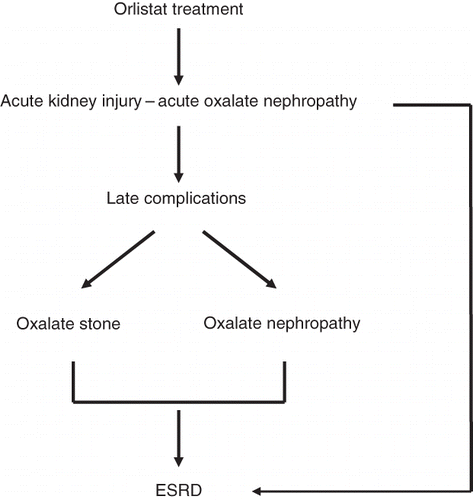
Epidemiologic studies have shown that obesity is also associated with increased risk of renal stone formation.Citation7 Importantly, bariatric surgery (in particular, gastric bypass surgery) has been shown to frequently cause hyperoxaluria with increase risk of stone formation and even oxalate nephropathy.Citation16 Orlistat is mainly prescribed to obese individuals and in most occasions with two or more of the features of metabolic syndrome. It is not yet clear whether administration of orlistat will add more to the risk of formation of oxalate stones ().
Furthermore, in one case report, administration of orlistat was associated with acute tubular necrosis (ATN) as a consequence of oxalate-induced oxalate nephropathy.Citation17 The same authors reviewed 855 renal biopsies for ATN and crystals carried between 1997 and 2007. Calcium oxalate crystals were found in two patients with unexplained ATN treated with orlistat at that time.Citation18 Singh et al. reported a case of orlistat-induced acute kidney injury secondary to acute oxalate nephropathy in a patient with underlying chronic kidney disease (CKD). Administration of orlistat was associated with acute kidney injury and urine sediment showed abundant calcium oxalate crystals and increased 24-hour urine oxalate concentration. Kidney biopsy showed deposition of calcium oxalate crystals within tubular lumens, consistent with acute oxalate nephropathy. Orlistat therapy was discontinued, and oral fluid intake was increased. A second kidney biopsy and a repeated 24-hour urine oxalate were free from oxalate with significant improvement in renal function.Citation19 It is likely to suggest that in an individual with pre-existing renal disease and concurrent administration of orlistat, any deterioration in renal function may warrant the need to exclude the possibility of orlistat-induced oxalate nephropathy ().
CONCLUSION
Orlistat is widely used as anti-obesity medication and can lead to 10% reduction in total body weight. Importantly, it can also reduce incidence of diabetes in individuals, with impaired glucose tolerance. In very small number of individuals especially those with pre-existing renal disease it may cause deterioration in renal function. This may include acute kidney injury due to acute oxalate nephropathy and chronic complications like oxalate nephropathy and renal stone. One important question with current increase in numbers of bariatric surgery is whether the previous orlistat treatment may also increase the risk of oxalate nephropathy. Currently, orlistat is safe and widely used anti-obesity medication. However, further research is needed to elicit the best means of detecting and treating renal complications associated with orlistat therapy.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of this paper.
REFERENCES
- Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–1574.
- Ahmed MH, Byrne CD. Metabolic syndrome, diabetes & CHD risk. In: Packard CJ, ed. The Year in Lipid Disorders. Oxford, UK: Clinical publishing; 2007:3–26.
- Ahmed MH, Khalil A, Osman MM. Nitric oxide as treatment for an emerging epidemic of obesity-related glomerulopathy. Diabetes Res Clin Pract. 2006;74(2):207–208.
- Hall JE, Jones DW, Kuo JJ, da SA, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5(5):386–392.
- Wesson DE, Kurtzman NA, Frommer JP. Massive obesity and nephrotic proteinuria with a normal renal biopsy. Nephron. 1985;40(2):235–237.
- Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001;59(4):1498–1509.
- Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183(2):571–575.
- Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11(3):188–195.
- Bray GA. Lifestyle and pharmacological approaches to weight loss: Efficacy and safety. J Clin Endocrinol Metab. 2008;93(11 Suppl. 1):S81–S88.
- Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161.
- MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van NM, Macdougall IC. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55(1):69–76.
- Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31(4):927–949.
- Lindsjo M. Oxalate metabolism in renal stone disease with special reference to calcium metabolism and intestinal absorption. Scand J Urol Nephrol Suppl. 1989;119:1–53.
- Ferraz RR, Tiselius HG, Heilberg IP. Fat malabsorption induced by gastrointestinal lipase inhibitor leads to an increase in urinary oxalate excretion. Kidney Int. 2004;66(2): 676–682.
- Sarica K, Akarsu E, Erturhan S, Yagci F, Aktaran S, Altay B. Evaluation of urinary oxalate levels in patients receiving gastrointestinal lipase inhibitor. Obesity (Silver Spring). 2008;16(7):1579–1584.
- Sinha MK, Collazo-Clavell ML, Rule A, Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72(1):100–107.
- Karamadoukis L, Shivashankar GH, Ludeman L, Williams AJ. An unusual complication of treatment with orlistat. Clin Nephrol. 2009;71(4):430–432.
- Karamadoukis L, Ludeman L, Williams AJ. Is there a link between calcium oxalate crystalluria, orlistat and acute tubular necrosis? Nephrol Dial Transplant. 2008;23(5): 1778–1779.
- Singh A, Sarkar SR, Gaber LW, Perazella MA. Acute oxalate nephropathy associated with orlistat, a gastrointestinal lipase inhibitor. Am J Kidney Dis. 2007;49(1):153–157.