Abstract
We present a case of interstitial nephritis and nephrogenic diabetes insipidus (NDI) in a patient treated with pemetrexed (500 mg/m2) for non-small cell lung cancer. Renal impairment and diabetes insipidus appeared after the first treatment cycle while he totally received four cycles of chemotherapy. There was not any significant myelosuppression and the patient was on regular supplementation with folic acid and vitamin B12. He was not on any other medications and he did not receive any nephrotoxic agents. Kidney biopsy showed acute tubular necrosis together with interstitial inflammatory infiltrate of mononuclear cells and interstitial fibrosis occupying 25% of the cortex. There was not any improvement of renal function after a 2-week trial of oral prednisone. In the present case report, we review the literature for pemetrexed-induced renal toxicity and the possible mechanisms involved.
INTRODUCTION
Pemetrexed disodium (Alimta, Eli Lilly and Co., Indianapolis, Indiana, USA) is a folate analog metabolic inhibitor approved for the treatment of advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC) and malignant pleural mesothelioma. Pemetrexed inhibits three enzymes involved in folate metabolism and DNA synthesis, resulting in the inhibition of purine and thymidine nucleotide and protein synthesis.Citation1
Cases of renal impairment have been reported after pemetrexed treatment, usually accompanied by significant myelosuppression that reversed after discontinuation of the drug. We report a biopsy-proven case of interstitial nephritis associated with nephrogenic diabetes insipidus (NDI) after pemetrexed therapy that did not respond to corticosteroid treatment and drug cessation.
CASE REPORT
A 57-year-old Caucasian man was presented with polyuria (>5 L/day), polydipsia, and clinical signs of mild dehydration (thirst, dry skin, blood pressure: 110/62 mmHg, pulse rate: 94/min). His medical history was significant for non-small cell lung cancer stage III B diagnosed 2 years ago. The patient had previously received carboplatin/paclitaxel, docetaxel/vandetanib in the setting of a clinical trial and then erlotinib without any significant improvement. Three months before his present admission to the hospital, the patient was started on treatment with pemetrexed (500 mg/m2 i.v., total dose 1 g, day 1), folic acid (350 mcg/day p.o.), vitamin B12 (1 g/60 days i.m.), and dexamethasone (4 mg bid for 3 days). After 3 consecutive 21-day cycles of chemotherapy, the disease had partially responded so a fourth cycle was given 20 days before presentation. On admission, blood chemistry revealed blood urea nitrogen of 15.35 mmol/L, a serum creatinine (sCr) of 300 μmol/L, plasma bicarbonate of 23 mmol/L, and serum sodium (sNa) of 152 mmol/L. Plasma osmolality was 309 mOsm/kg and plasma antidiuretic hormone (ADH) levels were 4.06 pmol/L (normal values corrected for osmolality: 3.69–11 pmol/L). Complete blood cell count, glucose, other electrolytes, hepatic enzymes, thyroid function, serum cortisol, and adrenocorticotropin hormone levels were all normal. In urine analysis, the specific gravity was 1005, pH 6, with no hematuria, leukocyturia, or dipstick proteinuria, and urine sodium was 25 mmol/L (fractional excretion of Na = 1.4). Ultrasound and computed tomography without intravenous contrast agent, showed normal kidneys and no disease progression in the abdomen. Magnetic resonance imaging (MRI) of the brain was normal. Upon review of patient's files, the baseline (before pemetrexed treatment) sCr was 83 μmol/L and sNa was 142 mmol/L, but after the first cycle of pemetrexed there was a gradual increase of sCr and sNa that have reached values of 133 μmol/L and 148 mmol/L, respectively, after the third cycle. However, the patient did not complain for any significant polydipsia or polyuria at that time, as he attributed his mild symptoms to the summer season. The patient underwent intravenous hydration and intranasal desmopressin without significant improvement, and a kidney biopsy was performed. The histological examination showed 17 glomeruli per section, 1 of which with global fibrosis and the remaining were normal. The interstitial tissue had focal edema with moderate infiltration from mononuclear inflammatory cells and fibrosis in approximately 25% of the cortex. The immunofluorescent examination showed negative expression of immunoglobulins IgG, IgA, IgM, complement fragments C3, C1q, and κ, λ light chains in glomeruli, tubules, and vessels. Electron microscopy examination was not performed ().
FIGURE 1. Interstitial infiltration from chronic inflammatory cells and mild tubulitis elements (arrows). (Hematoxylin and Eosin stain, original magnification ×250.)
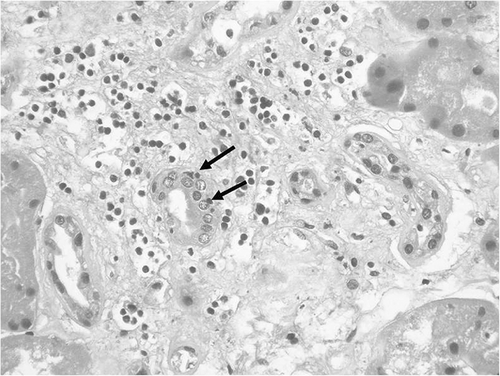
FIGURE 2. Interstitial infiltration from many inflammatory cells, mainly mononuclear cells. (Hematoxylin and Eosin stain, original magnification × 400.)
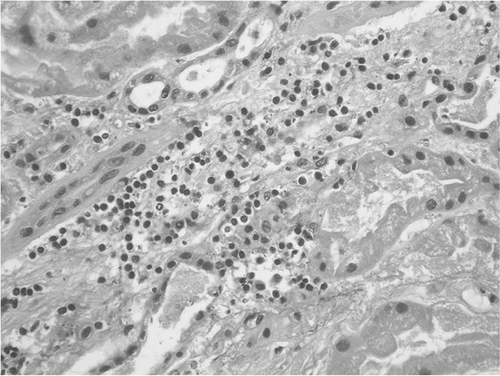
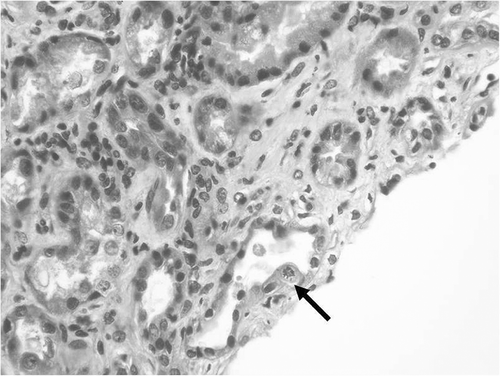
FIGURE 3. Acute tubular injury and regenerative lesions in epithelial cells with rare mitoses (arrow). (Hematoxylin and Eosin stain, original magnification × 400.)
The patient was treated with oral prednisone (60 mg/day) for 2 weeks, without any improvement in renal function (sCr: 283 μmol/L). sNa was controlled with hydration, low salt–low protein diet, and intranasal desmopressin, however polyuria persisted. One month later, the patient developed peritoneal carcinomatosis, his renal function deteriorated requiring dialysis; and he eventually died 2 months later.
DISCUSSION
Our patient developed irreversible acute kidney injury (AKI) related to acute tubular necrosis (ATN) and interstitial fibrosis, together with NDI, following treatment with pemetrexed for NSCLC. ATN seemed to be of nephrotoxic origin as there were no signs of prolonged significant renal hypoperfusion, despite the polyuria. Interstitial nephritis was attributed to pemetrexed, because the patient had not received any other potential causative agents. Carboplatin was used more than a year ago and in the meantime sCr, sNa, and urine analysis were all normal. The extent of fibrosis could be related to the delayed diagnosis and the additional doses of pemetrexed, as signs of renal impairment and NDI had already presented after the first dose. In addition, biopsy findings are similar with the findings of the only published report of pemetrexed-induced AKI in which kidney biopsy was performed. That patient developed ATN and chronic interstitial fibrosis after six cycles of pemetrexed, without, however, polyuria or hypernatremia as in our case.Citation2 In another patient who received pemetrexed (500 mg/m2) and cisplatin (75 mg/m2) for three cycles, then pemetrexed as a single agent, AKI appeared on the sixth cycle of pemetrexed.Citation3 Our patient experienced AKI after the first treatment cycle, indicating that toxicity was not related to a cumulative dose effect. Only few cases of AKI have been reported in some of the clinical trials of pemetrexed, usually not serious and reversible (). In a phase I study of 105 patients who received pemetrexed 600–1400 mg/m2, 3 of them discontinued therapy due to decreased creatinine clearance.Citation4 In the phase I pemetrexed maintenance therapy study (500 mg/m2) in 13 patients, creatinine clearance decreased significantly from 88 ± 21 mL/min at the end of the induction therapy to 77 ± 26 mL/min at the end of maintenance therapy.Citation5 In a phase II study of 92 patients, 1 patient who received pemetrexed 600 mg/m2 with a baseline creatinine level of 1.1 mg/dL experienced creatinine increase to 1.5 mg/dL at cycle 8 that persisted for more than 3 months after discontinuation. Another patient who received 900 mg/m2 developed renal failure that resolved 13 days after cessation of the drug.Citation6 In another phase III trial, in 121 fully vitamin-supplemented patients who received pemetrexed (500 mg/m2), 2 patients experienced grade 1 and 2 (reversible) decrease in creatinine clearance.Citation7 Since most of the trials had excluded patients with baseline creatinine clearance of <60 mL/min because of increased toxicity, little is known about the potential nephrotoxic effects of pemetrexed at a lower level of renal function.
TABLE 1. Summary of pemetrexed-induced renal toxicity cases in the literature
Hypernatremia and other electrolyte abnormalities have also been reported following administration of pemetrexed. In the clinical trial database of 519 patients, between December 1995 and February 2005, hypernatremia presented in 0.2% of the patients, but there was not any case of NDI.Citation8 In the literature, only one case of partially reversible acute renal failure, NDI, and renal tubular acidosis, concomitant with severe myelosuppression, has been reported in a patient who received three cycles of pemetrexed 500 mg/m2. However, a kidney biopsy was not performed and the AKI was thought to be secondary to ATN as she had been also exposed to intravenous contrast.Citation8 Diagnosis of NDI in our patient was based on polyuria concurrent with hypernatremia, low specific gravity and sodium in urine analysis, the normal plasma ADH, the normal hypothalamic–pituitary MRI, and the partial response of polyuria to desmopressin administration. The low–normal ADH concentration raised the possibility of central DI. However, hypothalamic, pituitary, and pituitary stalk appeared normal on MRI imaging and in the posterior pituitary a bright spot was present. We did not perform a deprivation test due to patient's dehydration on presentation. Moreover, the partial response of NDI to desmopressin is well described.Citation9 Pemetrexed was thought to be the cause of NDI, as cases of central DI have been described in metastatic lung cancer, but not NDI. The exact mechanism is not known; however, in other cases of drug-induced NDI the underlying urinary concentrating defect results from vasopressin-regulated water channel aquaporin-2 trafficking defects and/or reduced aquaporin-2 expression in collecting duct cells. Most cases are reversible after the discontinuation of the offending drug, but prolonged exposure can cause chronic irreversible changes and this maybe the case in our patient.Citation10 NDI has also been reported after administration of another antifolate agent, methotrexate, and the mechanism may be analogous.Citation11
The observed nephrotoxicity seems to be related to the mechanism of action of pemetrexed and its pharmacokinetics. Pemetrexed is eliminated as unchanged drug primarily from the kidney, by both tubular secretion and glomerular filtration with the former being the predominant mechanism for patients with normal renal function.Citation12 Folates and antifolates are reabsorbed at kidney proximal tubules via folate receptors and transported into cells.Citation13–15 We hypothesize that the accumulation of the drug intracellularly and the subsequent inhibition of purine and protein synthesis, result in tubular cytotoxicity and possibly secondary tubulo-interstitial inflammation leading to AKI. Our patient did not respond to corticosteroid treatment. Possible reasons include the prolonged renal impairment and the poor prognostic factors on kidney biopsy such as interstitial fibrosis and tubular atrophy. In addition, if the main mechanism of kidney injury is cytotoxic and not immunologic, then corticosteroids will be ineffective. Thymidine was used as an antidote for pemetrexed-related toxicity and initiated within 36 hours of pemetrexed infusion in one clinical report.Citation3 However, in our case, thymidine was not available and also our patient had received the last pemetrexed dose 20 days before presentation, so its efficacy would be uncertain, as pemetrexed's half-life elimination time ranges from 3.5 to 19.4 hours depending on renal function.Citation12
In summary, we presented a case of biopsy-proven, pemetrexed-induced interstitial nephritis associated with NDI that resulted in chronic renal failure. We suppose that renal tubular toxicity was responsible for the AKI and the tubular cell dysfunction of the distal nephron. Physicians should be aware of early signs of pemetrexed renal toxicity, as cessation of the drug and early treatment if needed, may preserve renal function.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- U.S. Food and Drug Administration: Center for Drug Evaluation and Research: Alimta, 2009. http://www. accessdata. fda.gov/drugsatfda_docs/Label/2009/021462s021lbl.pdf [accessed October 17, 2009].
- Michels J, Spano JP, Brocheriou I, Acute tubular necrosis and interstitial nephritis during pemetrexed therapy. Case Rep Oncol. 2009;2:53–56.
- Castro M. Thymidine rescue: An antidote for pemetrexed-related toxicity in the setting of acute renal failure. J Clin Oncol. 2003;21:4066–4069.
- Takimoto CH, Hammond-Thelin LA, Latz JE, Phase I and pharmacokinetic study of pemetrexed with high-dose folic acid supplementation or multivitamin supplementation in patients with locally advanced or metastatic cancer. Clin Cancer Res. 2007;13:2675–2683.
- van den Bogaert DP, Pouw EM, van Wijhe G, Pemetrexed maintenance therapy in patients with malignant pleural mesothelioma. J Thorac Oncol. 2006;1:25–30.
- Llombart-Cussac A, Martin M, Harbeck N, A randomized, double-blind, phase II study of two doses of pemetrexed as first-line chemotherapy for advanced breast cancer. Clin Cancer Res. 2007;13:3652–3659.
- Jassem J, Ramlau R, Santoro A, Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol. 2008;26:1698–1704.
- Vootukuru V, Liew YP, Nally JV. Pemetrexed-induced acute renal failure, nephrogenic diabetes insipidus, and renal tubular acidosis in a patient with non-small cell lung cancer. Med Oncol. 2006; 23:419–422.
- Moses AM, Scheinman SJ, Oppenheim A. Marked hypotonic polyuria resulting from nephrogenic diabetes insipidus with partial sensitivity to vasopressin. J Clin Endocrinol Metab. 1984;59:1044–1049.
- Garofeanu CG, Weir M, Rosas-Arellano MP, Causes of reversible nephrogenic diabetes insipidus: A systematic review. Am J Kidney Dis. 2005;45:626–637.
- Fernández-Espartero MC, Rodríguez M, de la Mata J. Methotrexate-induced nephrogenic diabetes insipidus: First case report. Rheumatology (Oxford). 2002;41:233–234.
- Mita AC, Sweeney CJ, Baker SD, Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol. 2006;24:552–562.
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: Biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417.
- Collins JF. Novel insights into intestinal and renal folate transport. Focus on ‘Apical membrane targeting and trafficking of the human proton-coupled folate transporter in polarized epithelia.' Am J Physiol Cell Physiol. 2008;294: C381–C382.
- Damaraju VL, Hamilton KF, Seth-Smith ML, Characterization of binding of folates and antifolates to brush-border membrane vesicles isolated from human kidney. Mol Pharmacol. 2005;67:453–459.