Abstract
Rationale: Cyclosporine A (CsA) leads to renal and liver injury, production of free radicals and nitric oxide (NO) deficiency. This study investigates the possible protective effects of trapidil and l-arginine against CsA-induced tissue injury. Objectives: Forty adult male Wistar rats (180 ± 20 g) were divided into five groups, eight animals in each. The first group served as control, second group served as CsA group, third group served as CsA + trapidil group, fourth group served as CsA + l-arginine group, and fifth group served as CsA + trapidil + l-arginine group. Kidney and liver functions, inflammatory mediators, cytokines, oxidant and antioxidant parameters as well as histopathological studies of renal and liver tissue were assessed in all groups. Main findings: CsA induced renal and hepatic dysfunction, which was confirmed by laboratory and histopathological examination. Administration of trapidil diminished the renal and liver injury and significantly attenuated the levels of serum creatinine, urea, aspartate aminotransferase (AST), alanine aminotransferase (ALT), interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and oxidative stress, while it significantly elevated the level of serum nitric oxide and the activity of antioxidative stress. l-Arginine gave the same trend as trapidil, but trapidil effect was more pronounced. Coadministration of trapidil + l-arginine significantly ameliorated the toxic effect of CsA, but did not differ significantly from the effect of trapidil alone. Conclusions: Treatment with trapidil or l-arginine diminished the renal and hepatic CsA-induced toxicity. However, the effect of trapidil was more pronounced. Therefore, treatment with trapidil alone may be the most economic and effective as a potential therapeutic agent in CsA injury.
INTRODUCTION
Cyclosporine A (CsA) is still one of the most widely used immunosuppressive drugs, especially in organ transplantation and the treatment of various autoimmune diseases.Citation1 This drug is extremely effective in preventing acute rejection of transplanted organs. However, despite the wide spectrum of CsA clinical uses, evidences have accumulated that it is associated with major adverse effects, the most important being renal and hepatic toxicity in humans and experimental animals.Citation2 The mechanisms by which CsA causes nephrotoxicity and hepatotoxicity are poorly understood; however, numerous studies have shown that CsA exposure enhances intracellular reactive oxygen/nitrogen species (ROS/RNS) production, suggesting that biochemical and physiological disturbances may result from oxidative and nitrosative stress.Citation3 Release of vasoconstrictor prostaglandins such as thromboxane A2 and activation of the sympathetic nervous system are the candidates of cyclosporine-induced vasoconstriction.Citation4 CsA leads to tissue injury, probably by causing the production of free radicals and resulting in nitric oxide (NO) deficiency.Citation5
Trapidil has a complex spectrum of biological activities. Several studies demonstrate its beneficial effects in various forms of tissue injury.Citation6 Trapidil is an anti-platelet-derived growth factor and vasodilator, which effect is achieved by competitive inhibition of phosphodiesterase.Citation7 It decreases platelet aggregation, which is attributed to its ability to increase prostaglandin synthesis,Citation8 inhibits platelet activation by reducing thromboxane synthetase,Citation9 and inhibits the proliferative effects of platelet-derived growth factor (PDGF) on vascular smooth muscle cells.Citation10 In addition, trapidil inhibits the release of monocyte chemoattractant protein-1 (MCP-1), thereby preventing the accumulation of macrophages following injured arterial wall,Citation11 and probably elevates nitric oxide (NO) levels which neutralize free superoxide anion radicals.Citation12
l-Arginine is an amino acid from which nitric oxide (NO) is synthesized. NO is considered to be an important regulator of renal vascular tone and a modulator of glomerular function under both basal and physiopathological conditions. It also plays an important role in hemodynamic alterations observed in acute renal failure.Citation13 The function of NO has been elucidated in a variety of pharmacological conditions, including inflammation, carcinogenesis and atherosclerosis. However, excess NO production causes oxidative damage to membrane lipids, DNA, proteins and lipoproteins.Citation14
Therefore, this study is designed to determine the possible protective effects of trapidil and l-arginine treatments against oxidative and nitrosative injury of kidney and liver induced by CsA.
MATERIALS AND METHODS
Animals
Adult male albino rats of Wistar strain (180 ± 20 g) were purchased from Egyptian Organization for Biological Products and Vaccines (Helwan, Egypt). The animals were housed in stainless steel wire–meshed suspended rodent cages under standard conditions of humidity, temperature (25 ± 2°C) and light/dark cycle (12/12 h). Rodent chow diet and water were allowed ad libitum. All animals received human care in compliance with guidelines of the Ethical Committee of Medical Research of National Research Centre, Egypt.
Drugs and dosage
The following drugs with dose levels as reported in the literature were used in this study:
CsA (Sandimmune, 50 mg/mL vial, Novartis Pharma AG, Basel, Switzerland): It was dissolved in olive oil so as to give a final concentration of 25 mg/mL.Citation2
Trapidil (Travisco) (MasterPharma, Italy): 100 mg/kg/day was orally gavage administered for 30 days.Citation15
l-Arginine (Sigma-Aldrich, St. Louis, USA): 25 g/L was supplemented in the drinking water ad libitum for 30 days.Citation16
Experimental design
Two weeks after acclimatization, the animals were divided into five groups, eight animals in each. These groups were as follows:
Control group: Rats were treated with olive oil, orally for 21 days, and served as normal control.
CsA group: Rats were orally administered with CsA (25 mg/kg/day) for 21 days.
CsA + trapidil group: Rats were treated with trapidil (100 mg/kg/day) for 30 days – 3 days before CsA administration, 21 days concomitant with CsA, and 6 days after.
CsA + l-arginine group: Rats were treated with l-arginine (25 g/L) supplemented in the drinking water ad libitum for 30 days – 3 days before CsA, 21 days concomitant with CsA, and 6 days after.
CsA + trapidil + l-arginine group: Rats were treated with CsA + trapidil + l-arginine as shown in groups 3 and 4.
Sampling
At the end of treatment period (30 days), animals were fasted overnight and then anesthetized using urethane (1.4 mL/kg).
Blood Sampling
Blood samples were collected via retro-orbital bleeding. Blood samples were collected in a dry centrifuge tube for serum separation, centrifuged at 3000×g for 15 min (4°C) and stored at −20°C as aliquots for further determinations of creatinine, urea, NO, interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), MCP-1, aspartate aminotransferase (AST), and alanine aminotransferase (ALT).
Tissue sampling
After blood collection, all animals were rapidly sacrificed and the liver and right kidney were removed by dissection, washed from blood by ice-cold isotonic saline. Part of each harvested kidney was shock-freezed in liquid nitrogen (–170°C) and stored at −20°C for further determination of superoxide anion (O2), susceptibility for lipid peroxidation, superoxide dismutase (SOD), catalase, and reduced glutathione (GSH). The other part of the right kidney together with the liver of each animal were stored in 10% formalin-saline at 4°C, and then processed for histopathological investigation.
Biochemical analysis
Creatinine concentration in serum was measured as described by HenryCitation17 using Diamond Diagnostics kits (Egypt), following the instructions of the manufacturer.
Serum urea was quantitatively determined using the QCA kit (Quimica Clinica Apilicada SA) with the modified Searcy method.Citation18
Serum NO measured as nitrite was determined using Griess reagent according to the method of Moshage et al.Citation19
Serum IL-1β was determined according to Zajkowska et al.Citation20 by solid-phase enzyme-linked immunosorbent assay (ELISA) using rat IL-1β kits (Biosource International, USA) and microtiter plate reader (Fisher Biotech, Germany).
Serum TNF-α was determined according to Chen et al.Citation21 by solid-phase ELISA using rat TNF-α kits (Biosource International, USA) and microtiter plate reader (Fisher Biotech, Germany).
Serum MCP-1 was determined according to Ono et al.Citation22 by solid-phase ELISA using rat MCP-1 kits.
Serum AST and ALT activities were determined using kits provided by Stanbio Laboratory (USA) according to Reitman and Frankel.Citation23 Tissue superoxide anion was determined according to the modified method of Hassoun and StohsCitation24 following the principle of Babior et al.,Citation25which measured the production of superoxide anion in tissue on the basis of reduction of cytochrome C.
Lipid peroxidation was determined according to Placer et al.,Citation26 which depends on the determination of thiobarbituric acid reactive substance (TBARS) content.
Total protein in tissue homogenate was determined according to Chromy and FischerCitation27 using diagnostic kits provided by Biocon (Germany) following the instructions of manufacturer.
The activity of tissue SOD was determined according to Marklund.Citation28 Tissue catalase activity was determined as described by Claiborne.Citation29
Principle
Histopathological investigation was done according to Prophet et al.Citation30 The fixed kidneys and livers in 10% neutral formalin were processed for histopathological examination. Paraffin sections 4–5 μm thick were stained with hematoxylin and eosin (H & E) for light microscopic examination.
The obtained data were statistically analyzed according to Steel and Torrie.Citation31 The results were presented at mean ± SD. The differences between mean values were determined by analysis of variance (ANOVA test), followed by Duncan's multiple rank testCitation32 using MSTAT (computer program).
RESULTS
Serum fractions
Feeding rats with 25 mg/kg CsA daily for 21 days caused a marked increase in the level of serum creatinine to 3.62 mg/dL (). There were significant increases in blood urea, IL-1β, TNF-α, and MCP-1 in the serum of rats supplemented with CsA as compared to the control (). The activity of AST and ALT also significantly increased due to feeding rats with CsA in comparison with the untreated rats (). Otherwise, supplementing rats with CsA resulted in a significant reduction in the level of serum nitric oxide. The value of serum NO reduced to 41.5% of the untreated rats (control). Oral administration of trapidil (100 mg/kg/day) 3 days before, 21 days concomitant with CsA administration, and 6 days after produced significant protection against renal and hepatic toxicity induced by CsA. The amelioration of renal and hepatic toxicity was evidenced by significant reductions in serum creatinine, urea, AST, and ALT concentrations in comparison with CsA group. In addition, trapidil supplementation significantly decreased the levels of IL-1β, TNF-α, and MCP-1, whereas it significantly increased NO in serum as compared to CsA group.
FIGURE 1. Effect of trapidil, l-arginine, and their combination on serum fractions of rats supplemented with 25 mg/kg CsA daily for 21 days *p < 0.05, **p < 0.01, ***p < 0.001 indicate the significance from the control.
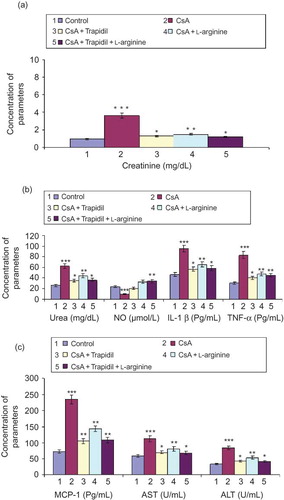
Rats supplemented with 25 g/L l-arginine in drinking water 3 days before, 21 days concomitant with CsA administration, and 6 days after produced significant protection against nephrotoxicity induced by CsA, which was manifested in significant reductions in serum creatinine and urea as compared to CsA group. In addition, l-arginine showed ameliorative effect for hepatotoxicity induced by CsA, which was manifested in significant reductions in the activity of serum AST and ALT. Moreover, l-arginine prevented the rise of IL-1β, TNF-α, and MCP-1 and the reduction of serum NO.
Rats supplemented with both trapidil and l-arginine show ameliorated nephrotoxicity and hepatotoxicity induced by CsA. However, there were insignificant differences between the effect of both trapidil + l-arginine and trapidil alone.
Tissue fractions
Data presented in revealed that CsA administration significantly elevated superoxide anion (O2) and lipid peroxidation activities by approximately three- and twofold, respectively, in renal tissue of rats as compared to the untreated rats (control). Otherwise, it significantly reduced the activity of SOD, catalase, and reduced GSH. However, superoxide anion and lipid peroxidation activities were significantly decreased by 45% and 41%, respectively, when rats were treated with CsA + trapidil compared to CsA alone. In addition, trapidil administration significantly increased SOD, catalase, and GSH activities by 73%, 143%, and 96%, respectively, as compared to CsA treatment alone.
FIGURE 2. Effect of trapidil, l-arginine, and their combination on renal tissue fractions of rats supplemented with 25 mg/kg CsA daily for 21 days *p < 0.05, **p < 0.01, ***p < 0.001 indicate the significance from the control.
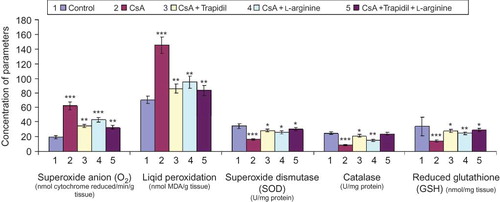
l-Arginine supplementation (fourth group) significantly lowered the values of superoxide anion activity by 31% and lipid peroxidation activity by 35% and significantly elevated the activity of SOD by 58% and both catalase and GSH by 74% when compared to rats treated with CsA alone.
Coadministration of both trapidil and l-arginine prevented the rise of oxidative stress parameters, that is superoxide anion and lipid peroxidation activities. Meanwhile, this treatment significantly elevated the activity of antioxidative stress parameters, that is SOD, catalase, and GSH. However, the difference between the effect of both trapidil + l-arginine and trapidil alone was not significant.
Histopathological studies
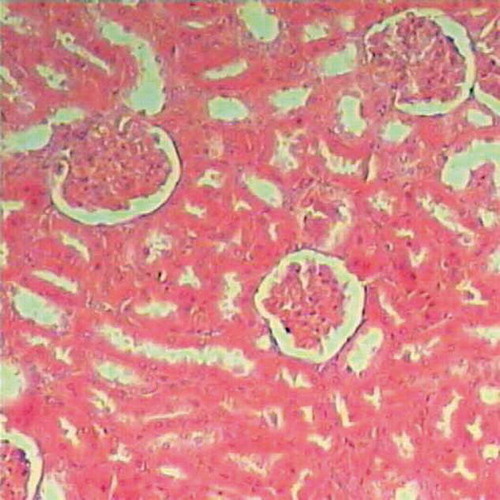
Histopathological examination of renal tissues of the untreated rats (control) showed normal renal parenchyma ().
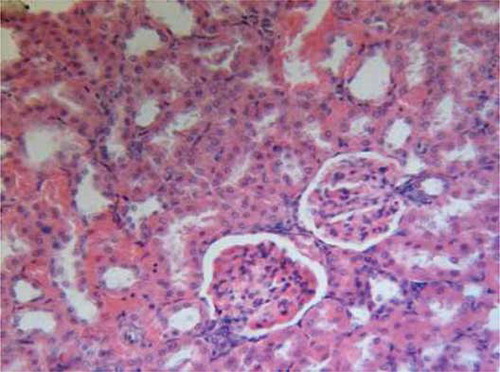
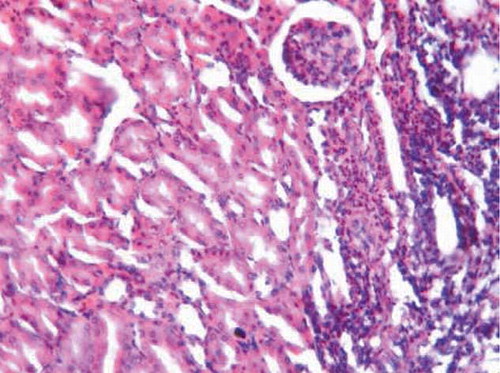
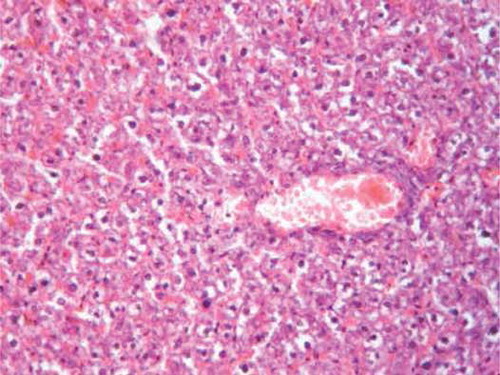
On the other hand, oral administration of 25 mg/kg/day CsA showed intense leukocytic aggregation replacing necrotic renal tubules (). Supplementing rats with trapidil showed protective effects against nephrotoxicity induced by CsA, which is manifested in only slight inflammatory infiltration (). Administration of l-arginine ameliorates kidney damage caused by CsA and showed only moderate hemorrhages in renal cortex and inflammatory infiltration (). Supplementing rats with both trapidil and l-arginine attenuated renal damage caused by CsA and showed mild infiltration ().
FIGURE A2. CsA group showing intense leukocytic aggregation replacing necrotic renal tubules ( H&E, ×200).
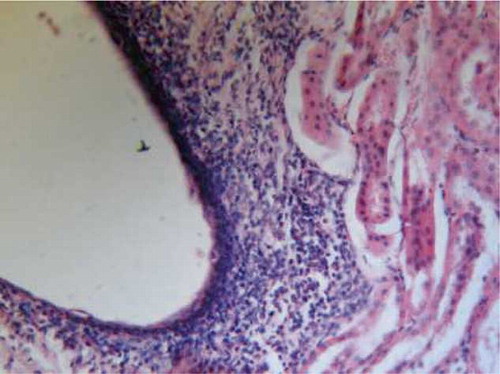
FIGURE A4. l-Arginine group showing moderate hemorrhages in renal cortex and inflammatory infiltration (H&E, ×200).
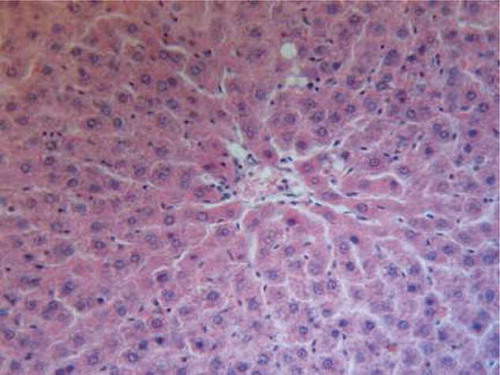
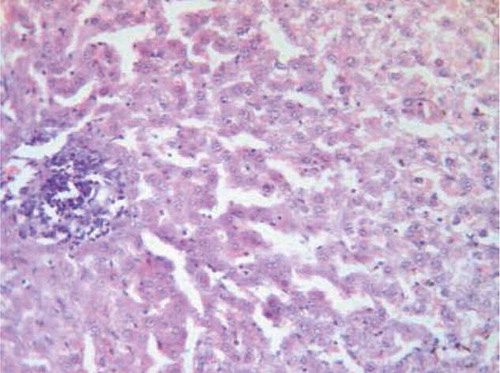
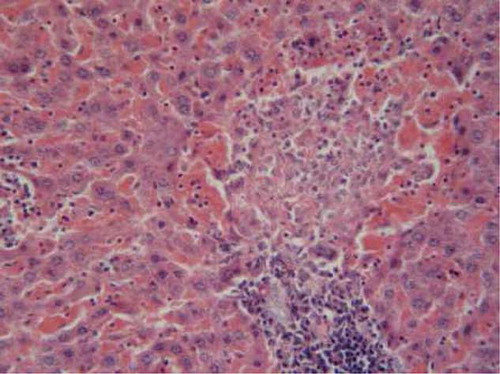
Specimens from liver tissue of control animals showed regular architecture and normal hepatic parenchyma (). However, examination of liver rats supplemented with 25 mg/kg/day CsA for 21 days indicated large necrotic areas surrounded by erythrocytes and congested blood vessels. (). The hepatic tissue of the rats supplemented with trapidil showed significant protection against the hepatotoxicity induced by CsA, which indicated mild degeneration of hepatic cells ().
FIGURE B1. Liver tissue of control animals showing regular architecture and normal hepatic parenchyma (H&E, ×200).
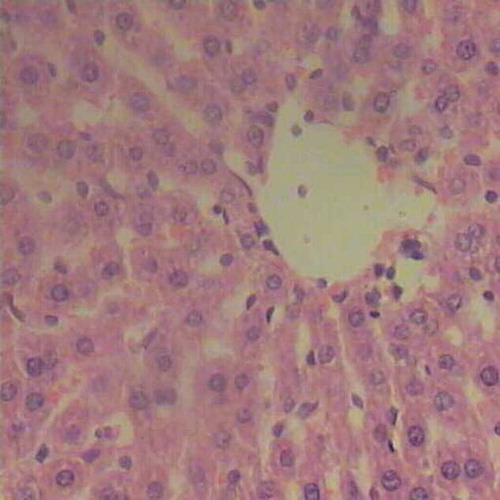
FIGURE B2. Liver of CsA group showing large necrotic areas surrounded by erythrocytes and congested blood vessels (H&E, ×200).
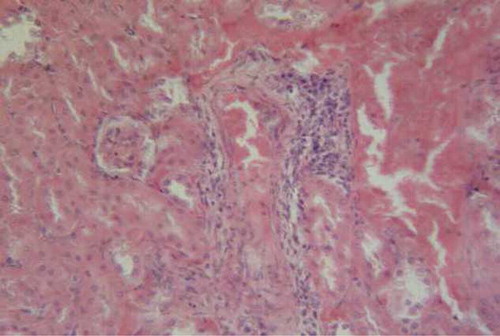
Administration of l-arginine ameliorated hepatic damage caused by CsA and showed mild degeneration and congested blood vessels (). Rats supplemented with both trapidil and l-arginine showed attenuated hepatic damage caused by CsA and mild interstitial lymphocyte aggregation in the hepatic parenchyma ().
DISCUSSION
CsA has been successfully used for the management of organ transplantations and autoimmune diseases for the last 25 years. Its use is limited by significant side effects such as nephrotoxicity, hepatotoxicity, hypertension and nervous system disorders.Citation33 The mechanisms leading to nephrotoxicity or hepatotoxicity are not fully understood.Citation34
The toxic renal effects of CsA observed in this study are manifested in a marked increase in the level of serum creatinine by 281% and urea by 100% as compared to the control, and confirmed by histopathological examination of renal tissue which showed intense leukocytic aggregation replacing necrotic renal tubules. The obtained results are in agreement with the finding of Amudha et al.Citation2
The hepatotoxicity induced by CsA in this study is characterized by increasing serum AST to 193% and ALT to 258% as compared to the control and confirmed by histopathological examination of hepatic tissue, which showed large necrotic areas surrounded by erythrocytes and congested blood vessels. These results are in accordance with the finding of Kurus et al.Citation5
CsA exposure enhances intracellular ROS/RNS production, suggesting that biochemical and physiological disturbances may result from oxidative and nitrosative stress.Citation3 Both ROS/RNS are considered as signaling mediators in the pathophysiology of CsA toxicity.Citation2
This study demonstrated that CsA exposure significantly elevated serum levels of IL-1β, TNF-α, and MCP-1. These results are in harmony with the previous findings of Ramesh and ReavesCitation35 and can be discussed on the basis that TNF-α and IL-1β are pro-inflammatory cytokines and often elevated in parallel. They stimulate the production of one another and act synergistically to stimulate the production of other cytokines and chemokines.Citation36 TNF-α itself could cause injury to renal epithelial cells where it stimulates the production of ROS and produces oxidative stress by sensitizing infiltrating leukocytes.Citation35 MCP-1 as a major chemotactic factor for monocytes is also induced by TNF-α and IL-1β in response to vascular injury and it is critical for the progression of atherosclerosis.Citation37
The obtained results showed that administration of CsA significantly decreased the level of serum NO. This result is in the same line as the finding of Kurus et al.Citation5 and may be due to the inhibition of endothelium-dependent vasodilatation.Citation38
The present results revealed that CsA induced a significant elevation in the level of superoxide anion and malondialdehyde (MDA) production and significantly reduced the activity of SOD, catalase, and reduced GSH. It is believed that reduced activity of one or more antioxidant systems because of the direct toxic effect of CsA causes an increase of lipid peroxidation and oxidative stress, and consequently renal and liver toxicity.Citation39
In this study, trapidil treatment diminished the nephrotoxicity of CsA, which is manifested in significant decrements in the level of serum creatinine and urea and confirmed by histopathological examination of renal tissue, which indicated only slight inflammatory infiltration in the renal tissue. Trapidil also attenuated the hepatotoxicity of CsA, which is manifested in significant reductions in the levels of serum AST and ALT and confirmed by histopathological examination of liver tissue, which showed mild degeneration of hepatic cells.
Administration of trapidil significantly elevated serum NO, similar to the finding of Colak et al.Citation40 It has been recently reported that trapidil as an antiplatelet and vasodilator drug can inhibit thromboxane A2 (TXA2) synthesis and stimulate prostacyclin (PGI2). Moreover, trapidil increases NO formation and/or diminishes its metabolism. NO and PGI2 can increase renal blood flow and glomerular filtration rate. It seems to act against nephrotoxicity by neutralizing free superoxide anion radicals.Citation12
The obtained results showed that trapidil significantly decreased the levels of serum, IL-1β, TNF-α, and MCP-1. These results are in accordance with the finding of Kato et al.Citation41
Deguchi et al.Citation42 suggested that this antiplatelet agent inhibits TNF-induced tissue factor expression on monocytes and may have a potent therapeutic value in patients with hypercoagulable state for its inhibitory property on the procoagulant activity of endothelial cells and monocytes.
Trapidil reduces the expression of MCP-1 antigen in the intima and media of the injured arterial wall.Citation11 The mechanism underlying the inhibition of MCP-1 accumulation by trapidil may be in part due to PDGF receptor antagonist function, inhibition of the mitogen-activated protein kinase cascade,Citation43 direct activation of protein kinase A, and alteration in prostaglandin synthesis.Citation44
Administration of trapidil induced significant reduction in the levels of superoxide anion and lipid peroxidation, while it significantly increased the activity of SOD, catalase, and GSH as compared to the treated rats with CSA alone. It is believed that increased activity of one or more antioxidant systems because of the direct effect of trapidil causes a decrease of lipid peroxidation and oxidative stress.Citation45 MDA is one of the most important end products of lipid peroxidation and is known to be an indicator of free radical damage and also indirectly of tissue damage.Citation46 Durak et al.Citation47 have shown that decrease in glutathione and accumulation of MDA are important mechanisms in CsA-induced toxicity.
It could be demonstrated that trapidil has protective effects and is a potent inhibitor of CsA-induced renal and liver toxicity via free radical scavenging effect and anti-inflammatory and antioxidant properties.Citation44
Nitric oxide has been implicated in the pathogenesis of inflammatory processes including CsA injury, and it participates in the regulation of microcirculation in several systems in both physiologic and pathologic settings.Citation48 The current results indicated that treatment with l-arginine attenuated the renal toxicity of CsA, which is manifested in significant reductions in the levels of serum creatinine and urea and confirmed by histopathological examination of renal tissue, which showed moderate hemorrhages in renal cortex and inflammatory infiltration. l-Arginine also diminished the hepatotoxicity of CsA, which is manifested in noticeable reductions in the levels of serum AST and ALT and confirmed by histopathological examination of liver tissue, which showed mild hydropic degeneration and congested blood vessels.
The contribution of NO in the pathogenesis of organ toxicity is still controversial. However, it has been demonstrated that exogenous nitric oxide precursor supplementation inhibited TNF-α and IL-1β, induced MCP-1 and mRNA expression in a dose-dependent manner, and also suppressed MCP-1 protein expression. Furthermore, it inhibited TNF-α and IL-1β, induced nuclear factor-κB (NF-κB) binding activity and suppressed the TNF-α-induced degeneration of inhibitory κB.Citation49
Supplementing rats with l-arginine in drinking water caused a significant reduction in pro-inflammatory cytokines TNF-α and IL-1β as well as inflammatory mediator MCP-1 levels. These results are in consistent with Saad et al.Citation50
Concerning the effect of l-arginine supplementation on the markers of oxidative stress, it is obvious that l-arginine decreased the levels of superoxide anion and MDA in renal tissue, while it increased SOD, catalase, and GSH. These results are in accordance with Mansour et al.Citation51
Tsao et al.Citation52 demonstrated that exogenous NO inhibits superoxide anion elaboration. NO may act by reducing intracellular oxidative stress, where NO scavenges the superoxide anion, although the product of this reaction, peroxynitrite anion, is in itself a highly reactive free radical.Citation53 However, it is possible that peroxynitrite anion could subsequently nitrosate sulfhydryl groups to form S-nitrosothiols. This class of molecules is known to induce vasodilation, inhibit platelet aggregation, and interfere with leukocyte adherence to the vessel wall.Citation54
Another mechanism by which NO may ameliorate oxidative stress is by terminating the autocatalytic chain of lipid peroxidation that is initiated by oxidation of low-density lipoprotein (LDL) or intracellular generation of oxygen-derived free radicals. Indeed, exogenous NO inhibits copper-induced oxidation of LDL cholesterol, causing a lag in the formation of conjugated dynes.Citation55 Finally, NO may directly suppress the generation of oxygen-derived free radicals by nitrosylating, and thereby inactivating, oxidative enzymes.Citation56
In addition, l-arginine greatly ameliorated reduced levels in glutathione peroxidase activity and GSH content in renal tissue and prevented the rise in lipid peroxides, indicating the possible protective effect of l-arginine against nephrotoxicity.Citation51
Diao et al.Citation57 suggested that NO has beneficial effect on the reperfusion injury in rat liver transplantation by supplementing the NO pathway. It was reported that NO may be protective in the hyporesponsive state. NO maintains a protective function with vasoconstricting effect against CsA. Some authors believe that the role of NO in injury-dependent oxidative stress is open for debate.Citation57 Aikio et al.Citation58 have reported that NO is a protective agent in case of oxidative injury but it both stimulates lipid peroxidation and acts as intermediate for protective reactions in the membranes. NO has been shown to inhibit the apoptosis of hepatocytes, which probably contributes to liver regeneration after partial hepatectomy. Nakanishi et al.Citation59 and Kurus et al.Citation5 demonstrated that l-arginine is a potent inhibitor of CsA-induced hepatoxicity via free radical scavenging effect.
l-Arginine may therefore be a beneficial remedy for CsA toxicity and can be used to improve the therapeutic index of CsA.
Supplementing rats with both trapidil and l-arginine attenuated nephrotoxicity induced by CsA, which manifested in significant reductions in serum creatinine and urea as compared to the untreated rats (control) and was confirmed by histopathological examination, which showed mild infiltration (Figure A5). This treatment also diminished the hepatotoxicity of CsA, which is manifested in significant decline in the level of serum AST and ALT and confirmed by histopathological examination of liver tissue, which indicates mild interstitial lymphocytes aggregation in the hepatic parenchyma (Figure B5). However, the effect of trapidil + l-arginine did not differ significantly from the effect of trapidil alone.
Trapidil + l-arginine treatment significantly raised the serum NO and decreased IL-1β, TNF-α, and MCP-1 as compared to CsA group. But there are insignificant differences between the effect of trapidil + l-arginine and trapidil alone.
Coadministration of trapidil + l-arginine significantly lowered superoxide anion, and lipid peroxidation, while it significantly raised the activity of SOD, catalase, and GSH as compared to CsA group, but did not differ significantly when compared to CsA + trapidil group. Therefore, treatment with trapidil may be the most effective as potential therapeutic agent in CsA injury.
CONCLUSION
Treatment with trapidil or l-arginine diminished the renal and hepatic toxicity induced by CsA exposure. However, the effect of trapidil was more pronounced. The protective effect of trapidil may be due to its multiple effects on lipid peroxidation and inflammatory mediator such as MCP-1 and cytokines such as TNF-α, whereas the protective effect of l-arginine could be via mechanisms that involve the production of NO.
Therefore, administration of either trapidil or l-arginine may be a beneficial remedy for CsA toxicity and can be used to improve the therapeutic index of CsA. However, treatment with trapidil alone may be the most economic and effective as a potential therapeutic agent in CsA injury.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Ponticelli C. Cyclosporine: From renal transplantation to autoimmune diseases. Ann. N.Y. Acad. Sci. 2005;1051:551–558.
- Amudha G, Josephino A, Varalakshmi P. Role of lipoic acid in reducing the oxidative stress induced by cyclosporine. Clinica Chimica Acta. 2007;372(1–2):134–139.
- Rezzani R. Exploring cyclosporine A- side effects and the protective role-played by antioxidants: The morphological and immunohistochemical studies, Histol. Histopathol. 2006;21:301–316.
- de Mattos AM, Olyael AJ, Bennett WM. Pharmacology of immunosuppressive drugs. Renal Failure. 1996;18:453–460.
- Kurus M, Esrefoglu M, Karabulut AB Sogutlu G, Kaya M, Otlu A. Oral l-arginine protects against cyclosporine – induced hepatoxicity in rats. Exp. Toxicol. Pathol. 2008;60(4–5):411–419.
- Atici A., Bozlu G, Turhan AH, The role of trapidil on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Human Develop. 2008;84(4):234–247.
- Joner M, Finn AV, Fab A, Vimani R. Pathology of drug eluting stents in humans: Delayed heating and late thrombosis risk. J Am Coli Cardial. 2006;48:193–202.
- Kawamura T, Tikitani T, Okajima Y, Effect of trapidil on prostacyclin generation of arterial wall. Prostaglandins Med. 1980;5(2):113–121.
- Suzuki Y, Yamaguchi K, Shimada S, Kitamure Y, Ohnishi H. Antithrombotic activity and the mechanism of action of trapidil. Prostaglandins Lekat Med. 1982;9(6):685–695.
- Cheng Y, Liu P, Chen H, Zng F. Antiproliferative effects of trapidil in vascular smooth cells are associated by inhibition of MAPK and P34 (cdc 2) activity. J. Cardiovasc. Pharmacol. 2000;35(1):1–6.
- Poon M, Cohen J, Siddiqui Z, Folton JT, Taubman MB. Trapidil inhibits monocyte chemoattractant protein-1 (MCP-1) and macrophage accumulation after ballon arterial injury in rabbits. Lab. Invest. 1999;79(11):1369–1375.
- Bagdtoglu C, Saray A, Surucu HS, Ozturk H, Tamer L. Effect of trapidil in ischemia reperfusion injury of peripheral nerves. Neurosurgery. 2002;51:212–220.
- Valdivielso JM, Blantz RC Acute renal failure: Is nitric oxide the bad guy? Antioxid. Redox. Signal. 2002;41(6):925–934.
- Steven Y, Jung T, Klotz LD, Schewe T, Grune T, Sies H. Protein modification elicited by oxidized low density lipoprotein (LDL) in endothelial cell. Protection by (–)-epicatechin. Free Rad. Biol. Med. 2007;42:955–970.
- Matsuno H, Stassen JM, Hoylaerts MF, Vermylen J, Deckmyn H. Fast and reproducible vascular neointima formation in the hamster carotid artery: Effect of trapidil and captopril. Thromb. Hemost. Dec. 1995;74(6):1591–1596.
- Tsao PS, McEvoy LM, Rexler HD, Butcher EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by l-arginine. Circulation. 1994;89(5):2176–2182.
- Henry RJ. Clinical Chemistry, Principles and Technics. 2nd ed. Harper and Row;1974:625.
- Searcy RI, Reardon JE, Foreman JA. Enzymatic serum urea determination. Am. J. Med. Technol. 1967;33:15–20.
- Moshage H, Kok B, Huizenga JR, Jansen PLM. Nitric and nitrate determination in plasma: A critical evaluation. Clin. Chem. 1995;4(6):892–896.
- Zajkowska JM, Hermanowska-Szpakowiez T, Pancewicz SA, Kondrusik M, Swlerzbinska R, Grygorezuk S. Serum concentration of TNF-α and IL-1β in patient with chronic hepatitis treated with interferon alpha: A preliminary report. Rocz. Akad. Med. Bialymst. 2002;47:276–286.
- Chen W, Jin W, Cook M, Weiner HL, Wahl SM. Oral delivery of group A streptococcal cell walls augments circulating TGF-beta and suppresses streptococcal cell wall arthritis. J. Immunol. 1998;161(11):6297–6304.
- Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, Matsushima SS. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79(2):195–203.
- Reitman S, Frankel S. The colorimetric method for determination of serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;28: 56–63.
- Hassoun EA, Stohs SJ. Cadmium induced production of superoxide anion and nitric oxide DNA single standard breaks and lactate dehydrogenase leakage in J. 774 A-1 cultures. Toxicology. 1996;112(3):219–226.
- Babior BM, Kipnes RS, Curnutte ST. Biological defense mechanisms, the production by leukocytes of superoxide potential bactericidal agent. J. Clin. Invest. 1973;52(3):741–744.
- Placer ZA, Cushman LL, Johnson BC. Estimation of lipid peroxidation (malondialdehyde) in biochemical systems. Anal. Biochem. 1966;16:359–365.
- Chromy V, Fischer J. Photometric determination of total protein in lipemic sera. Clin. Chem. 1977;23(4):754–756.
- Marklund SL. Pyrogallol autooxidation. In: Greenwold RA, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985:243–247.
- Clairborne A. Catalase activity. In: Greenwold RA, ed. Handbook of Methods for Oxygen Radical Research. Boca, Ration, FL: CRC Press; 1985:283–284.
- Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory Methods in Histotechnology. Washington, DC: American Registry of Pathology; 1992.
- Steel RDG, Torrie JH. Principles and Procedures of Statistics: A Biometrical Approach. 2nd Ed.Singapore: McGraw-Hill; 1984, 4th print.
- Duncan DB. Multiple range test and multiple F test. Biometrics. 1955;1:1–42.
- Grub S, Persohn E, Trommer WE, Wolf AE. Mechanisms of cyclosporine A-induced apoptosis in rat hepatocyte primary cultures. Toxicol. Appl. Pharmacol. 2000;163:209–220.
- Rezzani R. Cyclosporine A and adverse effects on organs: Histochemical studies. Prog. Histochem. Cytochem. 2004;39: 85–128.
- Ramesh G, Reaves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 2002;110(6):835–842.
- Banas B, Luckow B, Moller M, Chemokine and chemokine receptor expression in a novel human mesangial cell line. J. Am. Soc. Nephrol. 1999;10(11):2314–2322.
- Funakoshi Y, Ichiki T, Shimokawa H, Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38(1):100–111.
- Buyukafsar K, Yazar A, Dusmez D, Ozturk H, Polat G, Levent A. Effect of trapidil an antiplatelet and vasodilator agent on gentamicin-induced nephrotoxicity in rats. Pharmacol. Res. 2001;44(4):321–328.
- Turk G, Atessahin A, Sonmez M, Yuce A, Ceribasi AO. Lycopene protects against cyclosporine A-induced testicular toxicity in rats. Theriogenology. 2007; 67:778–785.
- Colak T, Polat A, Bagdatoglu O, Kanik A, Turkmenoglu O, Aydin S. Effect of trapidil in ischemia/reperfusion injury on rat small intestine. J. Invest. Surg. 2003;16(3):167–176.
- Kato Y, Tsuda T, Hosaka Y, Effect of trapidil on effect or functions of monocytes related atherosclerotic plaque. Eur. J. Pharmacol. 2001;428(3):371–379.
- Deguchi H, Takeya H, Wada H, Dilazep an antiplatelet agent inhibits tissue factor expression in endothelial cells and monocytes. Blood. 1997;90(6):2345–2356.
- Bonish D, Weber AA, Wittpoth M, Osinski M, Schror K. Antimitogenic effects of trapidil in coronary artery smooth muscle cells by direct activation of protein kinase A. Mol. Pharmacol. 1998;54:241–248.
- Nieder J, Claus P, Auguntin W. Effect of trapidil on PG 12 and TXA2 synthesis in human umbilical viens perfused in vitro. Prostaglandins. 1995;49:311–318.
- Ozaraz R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J. Gastroenterol. 2003;9:125–128.
- Canoruc N, Cieck R, Atamer A, Dursun M, Turgut C, Guneli E. Protective effect of vitamin E selenium and alloporinol against stress induced ulcer formation in rats. Turk J. Med. Sci. 2001;31:199–203.
- Durak H, Ozbek H, Elgun S. Cyclosporine reduces hepatic antioxidant capacity: Protective roles of antioxidants. Int. Immunopharmacol. 2004;4:469–473.
- Crooke JP, Tsao PS. Cytoprotective effects of nitric oxide. Circulation. 1993;88:2451–2454.
- Lee SK, Kim CS, Yang WS, Kim SB, Park SK, Park JS. Exogenous nitric oxide inhibitis tumor necrosis factor alpha or interleukin-1β induced monocyte chemoattractant protein-1 expression in human mesangial cells. Nephron. 2002;92(4):780–787.
- Saad SY, Najjar TA, Daba MH, Al-Rikabi AC. Inhibition of nitric oxide synthase aggravates cisplatin-induced nephrotoxicity effect of 2-amino-4-methylpyridine. Chemotherapy. 2002;48(6): 309–315.
- Mansour M, Daba MH, Gado A, Al-Rikabi A, Al-Majed A. Protective effect of l-arginine against nephrotoxicity induced by cyclosporine in normal rats. Pharmacol. Res. 2002;45(6): 441–446.
- Tsao PS, Wang B, Buitrage R, Shyy JY, Cooke P. Nitric oxide regulate monocyte chemotactic protein-1. Circulation. 1997;96: 934–940.
- Radi R, Becman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls: The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266:4244–4250.
- Stamler JS, Simon DL, Osborne JA, S-Nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992;89:444–448.
- Hogg N, Kalyanaramer B, Strnck A. Inhibition of low density lipoprotein oxidation by nitric oxide potential role in atherogenesis. FEBS Lett. 1993;334:170–174.
- Clancy RM, Leszczynska P, Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on NADP oxidase. J Clin Invest. 1992;90:1116–1121.
- Diao TJ, Yuan TY, Li YL. Immunologic role of nitric oxide in acute rejection of golden hamster for rat liver xenotransplantation. World J Gastroenterol. 2002;8:746–751.
- Aikio O, Pokela ML, Hallman M. Exhaled and nasal nitric oxide in mechanically ventilated preterm and term newborns. Acta Pediatr. 2002;91:1078–1986.
- Nakanishi H, Kaibori M, Teshima S, Yoshida H, Kwon AH, Kamiyama Y. Pirenldone inhibits the induction of I NOS stimulated by interleukin-1 beta at a step of NF-κB DNA binding in hepatocytes. J. Hepatol. 2004;41:730–736.