Abstract
Methylguanidine (MG), a small molecule among guanidine compounds, is a product of protein catabolism. The concentration of MG in the serum of uremic patients is nearly 80 times of that in the serum of normal people. The present study was designed to explore the toxic effect of MG on renal proximal tubular cells as well as the protective effect of antioxidants PGE1 and probucol against MG-induced apoptosis in renal proximal tubular cells. HK-2 cells were used as the subject. The cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. N-Acetyl-3-d-glucosaminidase (NAG) activity, malondialdehyde (MDA) content, and superoxide dismutase (SOD) activity were determined. Cell apoptosis was determined by flow cytometry (light scatter and propidium iodide/annexin V-FTC fluorescence) and by nuclear staining with Hoechst 33258. Cells were exposed to MG (0.25, 0.5, or 1 mmol/L), MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L) respectively for 24 h. MG induced a significant dose-dependent loss of cell viability. Both PGE1 and probucol improved the viability of MG-treated HK-2 cells. Cells showed apoptotic morphology (deepened stain, karyopyknosis, and apoptotic body) when exposed to 0.5 mmol/L MG for 24 h, and the apoptosis ratio was increased compared with the control. The presence of PGE1 or probucol significantly lowered the apoptotic ratio. Moreover, PGE1 or probucol notably decreased the MDA content and increased the SOD activity compared with when the cells were treated with MG only. The results of the present study clearly demonstrate that MG could promote apoptosis of renal proximal tubular cells in vitro. Both PGE1 and probucol could protect renal proximal tubular cells from MG-induced apoptosis.
INTRODUCTION
Methylguanidine (MG) is a metabolic product of creatinine (CTN) and some amino acid.Citation1–3 Studies revealed that the liver and the kidneys oxidize CTN into creatol, which is converted into MG.Citation4–7 Oxidation of CTN with active oxygen (e.g., hydrogen peroxide) was proven to generate MG, whereas scavengers of active oxygen (e.g., glutathione, sorbitol) considerably reduced MG synthesis.Citation8 MG is expelled mainly from the kidney and is considered as not only a neurotoxinCitation9–11 but also a critical uremic toxin. Some experiments revealed that animals with high-level MG displayed a lot of functional and pathological characteristic features of uremia.Citation12,Citation13 Another study showed significant reduction of the glomerular filtration rate, renal plasma flow, and renal blood flow after administration of MG to normal ratsCitation14 and rats with adenine-induced chronic renal failure.Citation15 Thus, MG has apparent toxic effects on kidney and causes deterioration of renal function, but the pathogenesis is still not very well understood. Considering that apoptosis is an important mechanism that contributes to the progressive loss of glomerular and tubular cells during the course of experimental chronic renal failure (CRF),Citation16 and our previous study showing that 1-methylhydantoin (another metabolic by-product of creatinine) has the ability to induce apoptosis in renal proximal tubular cells in vitro,Citation17 we hypothesized that MG impaired the renal function partly due to the induction of apoptosis in renal tubular epithelial cells.
Prostaglandin E1 (PGE1), known as an antioxidant, has wide-ranging cytoprotective effects for gastrointestinal mucosa,Citation18 liver,Citation19 and kidneys.Citation20 Many animal experiments demonstrated that PGE1 has antiapoptotic effect on various cells, such as neuronal apoptosis in spinal cord induced by sciatic constriction injury,Citation21 hepatocyte apoptosis in excessive hepatectomy,Citation22 endothelial progenitor cell (EPC) apoptosis,Citation23 and renal cell apoptosis induced by cisplatin.Citation24 Probucol, a lipid-lowering drug, is a synthetic antioxidantCitation25 that has been shown to inhibit oxidative stress-induced apoptosis of endothelial cells.Citation26 It has also been reported that pretreatment with probucol attenuates cardiomyocyte apoptosis induced by adriamycinCitation27 and in heart failure rat modelCitation28 and atrial apoptosis in prolonged pacing-induced atrial fibrillation dogs.Citation29 Research by Wang et al.Citation30 discovered that probucol protects the renal microvascular endothelial cells from advanced glycation end product-induced damage, but its protective effect against apoptosis in renal tubular epithelial cells has not been shown so far in any published document. On the basis of the aforementioned similarities, we hypothesized that PGE1 and probucol might have protective effects on MG-induced apoptosis in renal proximal tubular cells. Our in vitro model allowed us to assess the toxicity of MG to HK-2 cells and antiapoptotic effects of PGE1 and probucol on MG-induced HK-2 cell apoptosis at the cellular level.
MATERIALS AND METHODS
Cell culture and preparation
All reagents were obtained from Sigma-Aldrich Co. unless otherwise stated. HK-2 cells (American Type Culture Collection, Manassas, Virginia, USA), an immortalized human proximal tubule epithelial cell line, were grown and passaged in 75 cm2 cell culture flasks that contained 1640 medium supplemented with 5% fetal calf serum (FCS) and antibiotics (100 U/mL penicillin G, 100 mg/mL streptomycin). Cells were maintained at 37°C in an atmosphere of 95% air and 5% CO2. Near-confluent (80%) HK-2 cells were incubated with serum-free media for 24 h to arrest and synchronize cell growth. Then the media were exchanged for fresh serum-free media, treated with culture medium containing various concentrations of MG (0.25, 0.5, or 1 mmol/L), MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L). Cells incubated with culture medium without any drugs were set up as control group. After treatment for 24 h, the cells were harvested for detection.
Measurement of cell viability by MTT assay
The HK-2 cell was dispensed onto 96-well microplate at a concentration of 1 × 104 cell well−1. The multi-well plates were incubated at 37°C, 5% CO2 in air for 24 h with MG (0.25, 0.5, or 1 mmol/L), MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L), in 5% bovine serum-supplemented 1640 medium. All experiments were repeated at least twice, and each experimental condition was repeated at least in quadruplicate wells in each experiment. At the end of each treatment, cells were incubated with 1 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in fresh media and kept in a dark environment for 4 h at 37°C. Dimethyl sulfoxide (DMSO), 150 μL per well, was added under vigorous pipetting to dissolve the formazan crystals, as described previously.Citation31 Dye absorbance (A) in viable cells was measured at 570 nm, with 630 nm as a reference wavelength by the method of Sheerin et al.Citation32
NAG enzyme assay
N-Acetyl-3-d-glucosaminidase (NAG), a lysosomal enzyme that is a good marker of proximal tubular cell integrity, was evaluated. Cells were plated in 24-well plates to 70–80% confluence and then incubated for 24 h at 37°C. NAG was leaked into the supernatant and measured by Hitachi 707, as seen in the methods of Pugh et al.Citation33
Morphological observation of apoptotic cells
HK-2 cells were exposed for 24 h to MG (0.5 mmol/L) or media. To determine the cell morphologic changes, Hoechst staining was carried out according to the manufacturer's instructions (Beyotime, China). Briefly, cells were washed with phosphate-buffered saline (PBS), fixed with methanol/acetic acid (3:1) for 10 min, and then incubated with 0.5 mL Hoechst 33258 for 5 min. Observation was performed under a fluorescent microscope.
Apoptosis measurement by annexin V/PI staining
The HK-2 cell was dispensed onto a 6-well microplate at a concentration of 5 × 104 cell well−1. Eighty percent confluent HK-2 cells were incubated with serum-free media for 12 h to arrest and synchronize cell growth, supernatant was discarded, and annexin V/PI (propidium iodide, PI) staining (an apoptosis detection kit; Beijing Biosea Biotechnology Co., Ltd, China) was performed according to manufacturer's instructions, and then cells were analyzed using a flow cytometer (Becton Dickinson FACSAria, USA). Cells that showed annexin V-positive (with translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane) and PI-negative staining (with intact cellular membrane) were considered as early apoptotic cells.Citation34–36
Measurement of MDA content and SOD activity
Malondialdehyde (MDA) in HK-2 cell lysates was determined by the thiobarbituric acid (TBA) method and the activity of superoxide dismutase (SOD) was measured by the method of xanthine oxidase according to manufacturer's instructions. One SOD unit was defined as the amount of enzyme that inhibited formazan production by 50% at 37°C.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Comparisons were made using a one-way analysis of variance (ANOVA) followed by a multiple comparison procedure (Fisher least significant difference test), which was provided by SPSS 13.0 statistical software (SPSS, Chicago, Illinois, USA). A value of p < 0.05 was considered statistically significant.
RESULTS
Measurement of cell viability by MTT assay
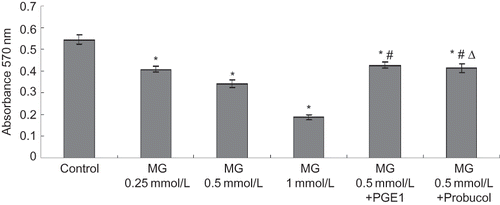
Evaluation of cell viability by the MTT assay demonstrated a significant (p < 0.01) dose-dependent reduction in MTT absorbance in HK-2 cells treated with MG for 24 h. Dye absorbance (A) of each concentration of MG was significantly (p < 0.01) lower than control. When PGE1 or probucol was added in MG group, the dye absorbance (A) increased significantly (p < 0.01), and the change level of PGE1 group was more obvious than that of the probucol group (p < 0.05). On the basis of the ability of viable cells to reduce MTT and the direct relationship between HK-2 cell number and MTT absorbance, MG induced a significant loss of cell viability, and the presence of PGE1 or probucol significantly lowered the MG-induced loss of cell viability (see ).
FIGURE 1. MG cytotoxicity and protective effects of antioxidants from cytotoxicity induced by MG in HK-2 cells. Six groups of HK-2 cells were treated with medium only, MG (0.25, 0.5, or 1 mmol/L), MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L), for 24 h. Cell viability was assayed by the MTT assay. Values are presented as mean ± SEM (n = 8). *p < 0.01 versus control, #p < 0.01 versus MG 0.5 mmol/L group, Δp < 0.05 versus MG (0.5 mMol/L) + PGE1 (2 μg/L) group.
NAG enzyme assay
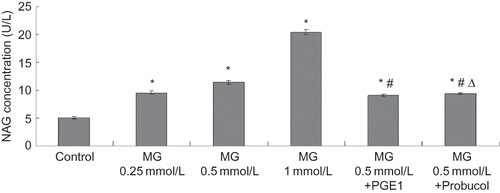
The exponentially growing HK-2 cell line was treated with MG at different concentrations of 0.25, 0.5, or 1 mmol/L, MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L). HK-2 cells treated with MG for 24 h displayed a significant (p < 0.01) dose-dependent increase in the supernatant concentration of NAG. Each concentration of MG was significantly (p < 0.01) greater than control. The presence of PGE1 or probucol significantly lowered the concentration of NAG (see ).
FIGURE 2. MG cytotoxicity and protective effects of antioxidants from cytotoxicity induced by MG in HK-2 cells. Six groups of HK-2 cells were treated with medium only, MG (0.25, 0.5, or 1 mmol/L), MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L), for 24 h. NAG release in the supernatant was determined. Values are presented as mean ± SEM (n = 8). *p < 0.01 versus control, #p < 0.01 versus MG 0.5 mmol/L group, Δp < 0.05 versus MG (0.5 mmol/L) + PGE1 (2 μg/L) group.
Morphological observation of apoptotic cells
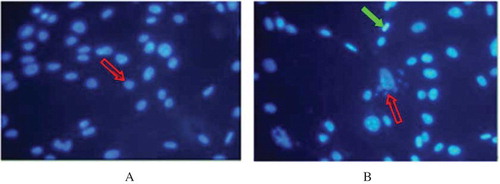
Hoechst staining showed that typical morphologic features of apoptosis, such as deepened stain, karyopyknosis, karyorrhexis, and apoptotic body (shown by the arrows in ), were observed after 24 h exposure to 0.5 mmol/L MG. No apoptotic morphology was observed in the control group (see ).
FIGURE 3. Epifluorescent micrographs showing Hoechst staining of DNA in HK-2 cells.
Note: Figure A stands for the HK-2 cells of normal control group; Figure B stands for the HK-2 cell of MG group, which were treated with 0.5mMol/L MG for 24 h. In figure A, shows normal nucleolus. Exposure to MG resulting in karyopyknosis and deepened stain (
) and the appearance of apoptotic bodies (
in figure B). Photographs taken at a magnification of × 400 (n = 4).
Effect of PGE1 and probucol on MG-induced HK-2 cell apoptosis by annexin V/PI staining
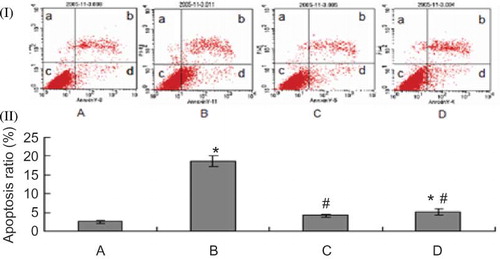
HK-2 cell apoptosis was assessed by annexin V/PI staining. As shown in , there was a low level of apoptotic cells in the control condition (2.473 ± 0.321)%. Exposure to MG for 8 h induced a significant increase in HK-2 cell apoptosis to (18.590 ± 1.413)% from (2.473 ± 0.321)% (p < 0.01). PGE1 or probucol pretreatment exerted a significant antiapoptotic effect: the presence of PGE1 or probucol significantly lowered the apoptotic ratio (4.183 ± 0.403)% or (5.030% ± 0.372)% versus (18.590 ± 1.413)% (p < 0.01), respectively, and the change level of PGE1 group was more obvious than that of the probucol group (p < 0.05).
FIGURE 4. Effect of PGE1 and probucol on MG-induced HK-2 cells apoptosis using flow cytometric analysis. Apoptosis ratio (%): (apoptosis cells/total cells) × 100%. (I) Flow cytometric analysis. (II) Densitometric analysis. A: control; B: 0.5 mmol/L MG; C: 0.5 mmol/L MG + 2μg/L PGE1; D: 0.5 mmol/L MG + 20 μmol/L probucol. Population a (annexin V−/PI+) is considered necrotic cells; population b (annexin V+/PI+) is considered late stage apoptotic cells; population c (annexin V+/PI−) is considered early apoptotic cells; population d (annexin V−/PI−) is considered normal HK-2 cells. Apoptotic ratio: *p < 0.01 versus control; #p < 0.01 versus 0.5 mmol/L MG group (n = 4).
Effects of PGE1 and probucol on oxidation in HK-2 cells
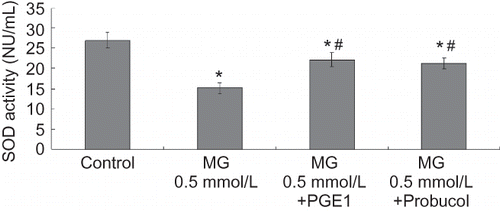
MDA levels were evaluated to estimate the degree of lipid peroxidation on cell culture. As shown in , MG caused significant increases in MDA levels. Meanwhile, PGE1 and probucol notably inhibited the increase in MDA content. SOD activity was used to detect the scavenging superoxide anion ability of cells. Total SOD activity diminished in the MG group compared to the control group at 24 h. PGE1 and probucol partially prevented the decrease in total SOD activity (see ).
FIGURE 5. Effects of PGE1 and probucol on MG-induced oxidation changes in HK-2 cells. Four groups of HK-2 cells were treated with medium only, MG 0.5 mmol/L, MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L), respectively, for 24 h. MDA content was determined. Values are presented as mean ± SEM (n = 4). *p < 0.01 versus control, #p < 0.01 versus MG 0.5 mMol/L group, Δp < 0.05 versus MG (0.5 mmol/L) + PGE1 (2 μg/L) group.
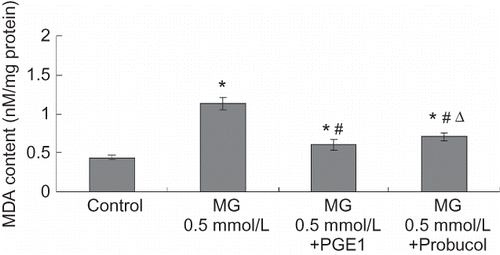
FIGURE 6. Effects of PGE1 and probucol on MG-induced oxidation changes in HK-2 cells. Four groups of HK-2 cells were treated with medium only, MG 0.5 mmol/L, MG (0.5 mmol/L) + PGE1 (2 μg/L), and MG (0.5 mmol/L) + probucol (20 μmol/L), for 24 h. SOD activity was determined. Values are presented as mean ± SEM (n = 4). *p < 0.01 versus control, #p < 0.01 versus MG 0.5 mmol/L group.
DISCUSSION
Animal experimentsCitation37 demonstrated that renal cell apoptosis takes part in the progress of glomerulosclerosis, glomerular atrophy, and renal interstitial fibrosis. Renal intrinsic apoptosis is an important pathological characteristic of chronic renal progressivity disease, which is one of the important factors associated with the progressive deterioration of renal function. Apoptosis of renal cells induced by some substances such as radiocontrast agents, hypoxia, albumin, aristolochic acid, adriamycin, and gentamicin has been widely reported.Citation38–43 However, the role of uremic toxins in inducing renal intrinsic apoptosis during progressive nephron deprivation in chronic renal insufficiency has been reported less frequently. Our present study was to examine whether the increased level of MG in the plasma of CRF patients induces apoptosis in renal proximal tubular cells and investigate some pharmacological interventions of this cell apoptosis in vitro.
In our experiment, when MG at different concentrations was added in media, the dye absorbance (A) decreased significantly and concentration of NAG in the supernatant increased significantly, which demonstrates that MG is toxic to proximal tubular cells. NAG is a sort of intracellular lysosomal enzyme EC3.2.1.30, which mainly exists in the renal proximal tubule and is one of the most sensitive marker enzymes of tubular epithelia injury.Citation44,Citation45 It is also discovered that after the MG effects on HK-2 cells, typical morphologic features of apoptosis, such as deepened stain, karyopyknosis, karyorrhexis, and apoptotic body, were observed by using Hoechst staining assay, which revealed that MG can promote HK-2 cells apoptosis in vitro. In addition, the annexin V/PI double staining method was employed to observe early apoptosis by flow cytometryCitation46 and revealed that cell apoptosis ratio in the MG group is significantly higher than in the normal control. This fact also confirms that MG has apoptotic effect on HK-2 cells. In fact, the increase in NAG in the supernatant is more compatible with the occurrence of tubular necrosis instead of apoptosis; thus, it suggests that both injuries could coexist in this model. Mori et al.Citation47,Citation48 demonstrated that extremely high level of guanidino compounds, including MG, generated reactive oxygen species (ROS) using an electron spin resonance (ESR) spectrometry with spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO). We also determined MDA content and SOD activity of HK-2 cells. MG increased MDA content and decreased SOD activity in vitro, which indicated that MG was effective in inducing oxidative process and inhibiting scavenging O2–. MDA is an important end product of lipid peroxidation and a widely used indicator of ROS production. The increase of MDA activity implied that the mechanism of MG-induced apoptosis may be somewhat related to its ability to produce ROS. However, the exact mechanism by which MG induces apoptosis remains unclear.
This experiment showed a protective role of PGE1 and probucol on MG-induced apoptosis in HK-2 cells. Experiment by Hsu et al.Citation43 proves that PGE1 can protect renal tubular cells from gentamicin-induced apoptosis, and also shows that PGE1 can block the activity of caspase-3 and decrease the generation of ROS. Another reportCitation49 shows that PGE1 can enhance the activity of SOD and stimulate adenylate cyclase (AC) system, thus triggering the activity of some key enzymes like Na, K, ATPase and blocking the activity of phosphodiesterase (PDE), which causes the opening of K+ channels in cell membranes, the increasing outflow of K+, and the decrease of intracellular Ca+ level, thus alleviating the overload of intracellular Ca+. In the meantime, PGE1 can protect the stability of cell membrane and cell organelle as well. In our study, when PGE1 was added in the media, the dye absorbance (A) increased significantly while the concentration of NAG significantly decreased in the supernatant, and annexin/PI assay also displayed a significant decrease of apoptosis ratio versus MG group, which reveals that PGE1 has a quite good antagonism effect on the apoptosis of renal proximal tubular cells induced by MG. What is more, after HK-2 cells were pretreated with PGE1, SOD activity increased and MDA content decreased compared with that of cells treated with MG only. SOD, as an important free oxygen radical scavenger in our body, eliminates superoxide anion and blocks lipid peroxidation chain reaction through disproportionating reaction. The finding suggested that the possible mechanism of the protective effect of PGE1 against MG-induced apoptosis in renal proximal tubular cells is related to its ability to elevate the oxidant scavenging ability and reduce the production of ROS.
Probucol is a hypolipidemic agent and has antioxidation effects. It has the ability of enhancing the activity of glutathione peroxidase (GSH-Px) and hydrogen peroxidase, inhibiting the generation of intracellular active oxygen and increasing its clearance.Citation50,Citation51 As a matter of fact, huge imbalance in the redox status that enhances the concentration of intracellular active oxygen is exactly the major mechanism of inducing cell apoptosis. GSH, as the most important intracellular thiol antioxidant, can directly eliminate hydroxyl radical (–OH) and superoxide anion (oxygen-derived free radicals) and eliminate H2O2 as a cofactor of GSH-Px.Citation52,Citation53 Our experiment reveals that after probucol co-treatment with MG on HK-2 cells, the dye absorbance (A) detected by MTT assay increased significantly while the concentration of NAG in the supernatant decreased significantly, Annexin/PI assay also displays a significant decrease of apoptosis ratio versus MG group; SOD activity increased and MDA content decreased compared with that of cells treated with MG only, which revealed that probucol can significantly attenuate the renal proximal tubular cells apoptosis induced by MG. The possible mechanism is related to its ability of direct clearance of free radical generated by MG.
In conclusion, cultured proximal tubular cells allowed us to observe and partly explain the toxic effect of MG on the kidney. MG-induced apoptosis in renal tubular epithelial cells is probably one of the important factors associated with the progressive deterioration of renal function. Antioxidants PGE1 and probucol can protect renal proximal tubular cells from MG-induced apoptosis in vitro partially because of their ability to attenuate the oxidative stress induced by MG, which lays the foundation for the further study of its precise mechanism and experiments in vivo. Our data suggested that PGE1 and probucol could be a potential therapeutic treatment for chronic progressive renal disease as there is still lack of an effective treatment today.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80(3):1107–1213.
- Perez G, Faluotico R. Creatinine: A precursor of methylguanidine. Experientia. 1973;29(12):1473–1474.
- Nagase S, Aoyagi K, Narita M, Tojo S. Biosynthesis of methylguanidine in isolated rat hepatocytes and in vivo. Nephron. 1985;40:470–475.
- Ienaga K, Nakamura K, Yamakawa M, The use of 13C-labelling to prove that creatinine is oxidized by mammals into creatol and 5-hydroxy-1-methylhydrantoin. J Chem Soc Chem Commun. 1991;509–510.
- Nagase S, Aoyagi K, Sakamoto M, Biosynthesis of methylguanidine in the hepatic microsomal fraction. Nephron. 1992;62:182–186.
- Aoyagi K, Akiyama K, Kuzure Y, Synthesis of creatol, a hydroxyl radical adduct of creatinine and its increase by puromycin aminonucleoside in isolated rat hepatocytes. Free Radical Res. 1998;29:221–226.
- Ozasa H, Horikawa S, Ota K. Methylguanidine synthase from rat kidney is identical to long-chain l-2-hydroxy acid oxidase. Nephron. 1994;68:279.
- Nagase S, Aoyagi K, Narita M, Tojo S. Active oxygen in methylguanidine synthesis. Nephron. 1986;44:299–303.
- Matsumoto M, Mori A. Effects of guanidine compounds on rabbit brain microsomal Na+, K+ ATPase. J Neurochem. 1976;27:635–663.
- D'Hooge R, Pei YQ, Marescau B, De Deyn PP. Convulsive action and toxicity of uremic guanidine compounds: Behavioral assessment and relation to brain concentration in adult mice. J Neurol Sci. 1992;112:96–105.
- Shimizu Y, Morimoto K, Kuroda S, Mori A. Sustained increase of methylguanidine in the rats after amygdala and hippocampal kindling. Epilepsy Res. 1995;21:11–17.
- Yokozawa T, Fujitsuka N, Oura H. Production of methylguanidine from creatinine in normal rats and rats with renal failure. Nephron. 1990;56(3):249–254.
- Giovannetti S, Biagini M, Balestri PL, Uraemia-like syndrome in dogs chronically intoxicated with methylguanidine and creatinine. Clin Sci. 1969;36(3):445–452.
- Yokozawa T, Oura H, Ienaga K, Nakamura K. Effects of creatinine, creatol and methylguanidine on renal function. Nippon Jinzo Gakkai Shi. 1992;34(9):973–977.
- Yokozawa T, Oura H, Ienaga K, Nakamura K. Comparison of renal effects of creatinine, creatol and methylguamdine in rats with adenine-induced chronic renal failure. Nephron. 1993;64(3):424–428.
- Thomas G, Yang B, Wagner B, Savill J, El Nahas A. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol Dial Transplant. 1998;13:2216–2226.
- Yang B, Liu D, Li CZ, 1-Methylhydantoin cytotoxicity on renal proximal tubular cells in vitro. Ren Fail. 2007;29(8): 1025–1029.
- Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77(3):433–443.
- Mokuno Y, Takano M, Matsuguchi T, Prostaglandin E(1) protects against liver injury induced by Escherichia coli infection via a dominant Th2-like response of liver T cells in mice. Hepatology. 1999;30(6):1464–1472.
- Abe K, Fujino Y, Sakakibara T. The effect of prostaglandin E1 during cardiopulmonary bypass on renal function after cardiac surgery. Eur J Clin Pharmacol. 1993;45(3):217–220.
- Kawamura T, Akira T, Watanabe M, Kagitani Y. Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology. 1997; 36(8):1023–1030.
- Ishibe A, Togo S, Kumamoto T, Prostaglandin E1 prevents liver failure after excessive hepatectomy in the rat by up-regulating cyclin C, cyclin D1, and Bclxl. Wound Repair Regen. 2009;17(1):62–70.
- Gensch C, Clever Y, Werner C, Regulation of endothelial progenitor cells by prostaglandin E1 via inhibition of apoptosis. J Mol Cell Cardiol. 2007;42(3):670–677. Epub 2007 Jan 9.
- Ikeguchi M, Maeta M, Kaibara N. Cisplatin combined with prostaglandin E1 chemotherapy in rat peritoneal carcinomatosis. Int J Cancer. 20001;88(3):474–478.
- Buckley MM, Goa KL, Price AH, Brogden RN. Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolaemia. Drugs. 1989;37(6):761–800.
- Aoki M, Nata T, Morishita R, Endothelial apoptosis induced by oxidative stress through activation of NFкB: Antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;381:48–55.
- Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antiopxid Redox Signal. 2001;3(1):135–145.
- Sia YT, Lapointe N, Parker TG, Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation. 2002;105(21):2549–2555.
- Li Y, Sheng L, Li W, Probucol attenuates atrial structural remodeling in prolonged pacing-induced atrial fibrillation in dogs. Biochem Biophys Res Commun. 2009;381(2):198–203.
- Wang Z, Liu ZC, Cui D, Yang XH. Effect of advanced glycation end products on injuring of rat renal microvascular endothelial cells and protective effect of probucol. Chinese Journal of Arteriosclerosis. 2008;16(2):111–611.
- Iwata M, Herrington J, Zager RA. Protein synthesis inhibition induces cytoresistance in cultured human proximal tubular (HK-2) cells. Am J Physiol. 1995;268(6 Pt 2):F1154–63.
- Sheerin N, Sacks S, Fogazzi G. In vitro erythrophagocytosis by renal tubular cells and tubular toxicity by hemoglobin and iron. Nephrol Dial Transplant. 1999;14:1391–1397.
- Pugh D, Leaback EA, Walker PG. Studies on glucosaminidase: N-acetyl-3-d-glucosaminidase in rat kidney. Biochem J. 1957;65:464–468.
- Darzynkiewicz Z, Bruno S, Del Bino G, Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808.
- Karwatowska-Prokopczuk E, Nordberg JA, Li HL, Engler RL, Gottlieb RA. Effect of vacuolar proton ATPase on pHi, Ca2+, and apoptosis in neonatal cardiomyocytes during metabolic inhibition/recovery. Circ Res. 1998;82:1139–1144.
- Martin SJ, Reutelingsperger CP, McGahon AJ, Early redistribution of plasma membrane phosphatidylserine is a general future of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Sb1. J Exp Med. 1995;182:1545–1577.
- Yang X, Shen YC, Zhu ZH, Deng AG, Liu JS. Apoptosis and expression of apoptosis-related genes in kidneys of the rats 5/6 nephrectomy. Chin J Phys Med Rehabil. 2007;23(5):916–920.
- Garofalo AS, Borges FT, Dalboni MA, Pavão dos Santos OF. Reactive oxygen species independent cytotoxicity induced by radiocontrast agents in tubular cells (LLC-PK1 and MDCK). Ren Fail. 2007;29(2):121–131.
- Shen Q, Yang XW, Wang Q, Relationship of S period apoptosis excessiveness with G1/S renal tubular epithelial cells exposed hypoxia. J Nephrol Dialy Transplant. 2005;14(5):427–431.
- Luo CX, Zhang Y, Zhang JE, Li T. Albumin induces apoptosis in rat tubular epithelial cells. J Chinese Physician. 2006;8(5):577–579.
- Wang W, Zhang J. Protective effect of erythropoietin against aristolochic acid-induced apoptosis in renal tubular epithelial cells. Eur J Pharmacol. 20087;588(2–3):135–140.
- Chen CH, Lin H, Hsu YH, The protective effect of prostacyclin on adriamycin-induced apoptosis in rat renal tubular cells. Eur J Pharmacol. 2006;529(1–3):8–15.
- Hsu YH, Chen CH, Hou CC, Prostacyclin protects renal tubular cells from gentamicin-induced apoptosis via a PPARalpha-dependent pathway. Kidney Int. 2008;73(5):578–587.
- Laina A, Schwartz D. Renal tubular and molecular events in acute renal failure. Nephron. 1994;68(4):415–841.
- Whiting PH, Peterson J, Power DA. Diagnostic value of urinary N-acetyl-β-d-glucosaminidase, its isoenzymes and the fractional excretion of sodium following renal transplantation. J Clin Chim Acta. 1983;130:369.
- Rosales-Torres AM, Avalos-Rodriguez A, Vergara-Onofre M, Multiparametric study of atresia in ewe antral follicles: Histology, flow cytometry, internucleosomal DNA fragmentation, and lysosomal enzyme activities in granulosa cells and follicular fluid. Mol Reprod Dev. 2000;55(3):270–281.
- Mori A, Kohno M, Masumizu T, Packer L. α-Guanidinoglutaric acid as a free radical generator. Biochem. Mol. Biol. Int. 1995;37:371–374.
- Mori A, Kohno M, Masumizu T, Noda Y, Packer L. Guanidino compounds generate reactive oxygen species. Biochem. Mol. Biol. Int. 1996;40:135–143.
- Noda U, Murakami S, Meves MH. The action of prostaglandins on ion channels. Curr Neuropharmacol. 2006; 4(1):41–57.
- Mantha SV, Kalra J, Prasad K. Effects of probucol on hypercholesterolemia-induced changes in antioxidant enzymes. Life Sci. 1996;58(6):503–509.
- Iliskovic N, Hasinoff BB, Malisza KL, Mechanisms of beneficial effects of probucol in adriamycin cardiomyopathy. Mol Cell Biochem. 1999;196(1–2):43–49.
- Pollman MJ, Hall JL, Gibbons GH. Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury. Influence of redox state and cell phenotype. Circ Res. 1999;84(1):113–121.
- Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17(8):4792–4800.