Abstract
Objective: Left ventricular hypertrophy (LVH) and atherosclerosis are frequently observed in uremic patients and they have appeared as an independent predictor of cardiovascular morbidity and mortality. The aim of this study was to compare the effects of ramipril and amlodipine on left ventricular mass index (LVMI) and carotid intima-media thickness (CIMT) in nondiabetic hypertensive hemodialysis patients. Methods: A total of 112 hemodialysis (HD) patients were included in this study. Patients were randomly allocated to receive ramipril or amlodipine for 1 year. Blood pressure (BP) measurements, LVMI, and CIMT were assessed at baseline and 6-month intervals. Biochemical parameters and inflammatory markers were also determined at the initiation and during the study period. Results: Similar BP decrease was observed in treatment groups. During follow-up, LVMI and CIMT progressed likewise in both treatment groups despite BP control. However, subgrouping analyses due to the pattern of left ventricular geometry showed that LVMI in patients with eccentric LVH increased, whereas LVMI decreased in subjects with concentric LVH under antihypertensive treatment. Discussion: BP control with ramipril or amlodipine could not provide adequate protection for development or progression of atherosclerosis and eccentric type of LVH in nondiabetic HD patients.
INTRODUCTION
Hypertension is the most common complication in hemodialysis (HD) patients.Citation1 Long-standing high blood pressure (BP) is associated with cardiovascular complications, which are one of the most common causes of mortality in patients under HD treatment. Current dialysis practice showed that the majority of HD patients will need antihypertensive drugs to reach target BP values. All classes of antihypertensive drugs can be used in HD patients. At that point, an important question is whether various antihypertensive drugs affect cardiovascular system to a similar extent. Morbidity and mortality trials have not been conducted in HD patients with hypertension; however, some guidance on the use of antihypertensive therapy can be made based on surrogate end points. Carotid intima-media thickness (CIMT) and left ventricular mass index (LVMI) were well-defined surrogate markers for cardiovascular diseases. Moreover, they have appeared as independent predictors of cardiovascular morbidity and mortality in HD patients.Citation2,Citation3 Long-term HD patients who had already attained dry weight and normotension still had substantial cardiovascular remodeling, including increased LVMI and CIMT.Citation4 These findings may imply that optimal dialysis alone does not nullify the increased cardiovascular risk in patients with end-stage renal disease (ESRD). Other interventions may be required to protect these patients from cardiovascular complications. The probability that certain antihypertensive drugs, such as calcium channel blockers (CCBs) and angiotensin-converting enzyme (ACE) inhibitors, exert an anti-atherosclerotic and antileft ventricular hypertrophy action that is at least partly independent of the BP-decreasing effect is supported by evidence obtained from studies in non-uremic patients.Citation5,Citation6 However, to date, there are not enough randomized clinical trials to assess the comparative effects of these agents on LVMI and atherosclerosis in nondiabetic HD patients. The geometric pattern of the left ventricle has also been associated with different cardiovascular outcomes in both the general population and hypertensive non-uremic subjects. However, the effect of LV geometry in response to antihypertensive therapy with different agents in HD patients is not identified.
This study attempts to answer an important question for the treatment of HD patients, namely if either ramipril or amlodipine is superior to the other with regards to treatment of LVH or atherosclerosis.
PATIENTS AND METHODS
Study population and study design
Between 2004 and 2006, about 112 hypertensive HD patients who were followed up in our nephrology department were randomized to either ramipril or amlodipine in a parallel group comparative study. Inclusion criteria for the trial were presence of ESRD treated with HD, evidence for hypertension (predialysis systolic and/or diastolic BP > 140/90 mmHg (predialysis office BP), and/or presence of an antihypertensive medication). Exclusion criteria were as follows: diagnosis of chronic infectious disease, coronary artery disease, myocardial infarction or cerebrovascular accident in the past 12 months, known intolerance to study medication, evidence for severe hepatic disease, use of immunosuppressant or nonsteroidal anti-inflammatory drugs (NSAIDs), congestive heart failure, the presence of a malignant disease or noncompliance of the subjects, valvular heart disease, other vascular diseases, and diabetic nephropathy (diabetes mellitus is a major independent promoter of atherosclerosis).
All patients' treatment was regular bicarbonate standard dialysis that lasted 4–5 h, three times per week (mean ± SD duration, 42.8 ± 8.1 months), with dialyzer surface area ranging from 1.3 to 1.8 m2. All the patients were treated using synthetic membranes (mainly polyamide and polysulfone). Dialysis period and dialyzer type were prescribed individually based on a urea-kinetic model to maintain Kt/V greater than 1.2. Dry body weight was evaluated on a clinical basis with the aid of chest radiographs performed every 3 months to assess cardiothoracic index. Vascular access was via arteriovenous fistula in all 112 patients.
All patients were administered intermittent subcutaneous individually adjusted doses of recombinant human erythropoietin to keep hemoglobin levels between 10 and 12 g/dL. Most patients were also treated with oral or intravenous calcitriol supplements and calcium carbonate or acetate and sevelamer (n = 8) tablets, if required.
The study was carried out in accordance with the Declaration of Helsinki (1989) and informed consent was obtained for all patients. Study protocol has been approved by Hacettepe University ethic committee on human research. Antihypertensive therapy was discontinued for at least 4 weeks prior to the beginning of the study. After the washout period, patients were randomly allocated to receive doses of 5 mg ramipril or 5 mg amlodipine per day. The randomization ratio was 1:1. If the target systolic/diastolic BP was not <140/90 mmHg (pre-dialysis office BP), the dose of ramipril or amlodipine could be increased to 10 mg once daily. Study medication use was recommended in the morning. The maximum doses were 10 mg/day for ramipril and 10 mg/day for amlodipine. After titration periods (4 weeks), mean 24 h ambulatory BP measurements within the following dialysis-free day were recorded. If the medication with maximum doses of ramipril or amlodipine was not sufficient to reach the target BP of less than 135/85 mmHg (24 h ambulatory BP), these patients were excluded from the study. The duration of the study was 12 months. Follow-up visits were scheduled at 3-month intervals. Follow-up visits included clinical assessment and measurement of 24 h ambulatory BP and routine laboratory tests. All patients underwent two-dimensional guided M-mode echocardiography and B-mode carotid ultrasonography at baseline, at 6 and 12 months of follow-up. All patients on treatment were evaluated for drug safety, tolerance, and efficacy in HD setting for 12 months. Serious adverse events were defined as withdrawal for any medical reason, death, and any abnormal laboratory value associated with signs or symptoms or necessitating treatment. All alterations in medication and any adverse events were recorded. The end points for this study were the alteration in LVMI and CIMT from baseline to follow-up after 12 months of treatment with amlodipine versus ramipril in hypertensive HD patients.
Blood pressure measurements
Ambulatory BP was measured over a 24 h period by the oscillometric method using an automatic noninvasive recorder (Spacelab Inc., Redmond, Washington, USA) on the following day after dialysis. The monitor was programmed to measure BP at 15 min intervals between 8:00 a.m. and 10:00 p.m. and at 30 min intervals between 10:00 p.m. and 8:00 a.m. During measurement, patients performed their usual regular daily activities. Measurements were only included if more than 85% of the readings were successful. Mean 24 h systolic BP, diastolic BP, and mean arterial pressure (MAP) were recorded in all patients.
ECHOCARDIOGRAPHY
A Vingmed CFM 750 ultrasonographic device system (GE Vingmed Sound, Horten, Norway) with a duplex mechanical annular array probe was used. Signals from the last 10 s are stored in an internal replay memory where they can be recalled or transferred to external computers. The patients were examined in the lateral recumbent position after 30 min of rest. Dimensions were measured by M-mode echocardiography. The same examiner performed recordings at baseline and on every follow-up at 6-month intervals. In our laboratory, the intra-observer variability is below 10% for LVMI (4.6 ± 1%). Recordings from each patient were analyzed at the same time to ensure consistent measurement technique using a devoted analysis program (Echopac, GE Vingmed Sound). Dimensions and chamber function assessed by ejection fraction (EF) of the left ventricle were measured using the recommendations of the American Society of Echocardiography.Citation7 The two-dimensional left ventricular endocardium was traced at end diastole and peak systole in cine loops of apical four-chamber and two-chamber views, and the correct positions of the tracings were controlled by running the cine loops.
Left ventricular mass (LVM) was calculatedCitation7 as
where LVIDd is left ventricular diastolic dimension, PWTd the diastolic dimension of posterior wall, and SWTd the diastolic dimension of interventricular septum, in centimeters. LVM was normalized for body surface area for calculation of LVMI.
LVH was identified by validated gender-specific partition values of LV mass index, ≥116 g/m2 in men and 96 g/m2 in women.Citation7 Relative wall thickness (RWT) was calculated by the formula (2 × posterior wall thickness (PWTd)/left ventricular internal dimension (LVIDd)). LV geometry was assessed from LV mass index and RWT in combination. Concentric geometry was considered present if RWT was ≥0.42.Citation7
CAROTID ULTRASONOGRAPHY
The IMT was measured by one trained radiologist without knowledge of the clinical data. IMT, defined as the distance between the media–adventitia interface and the lumen–intima interface, was measured using a duplex ultrasound system with a 7.5 MHz scanning frequency in the B-mode, pulsed Doppler mode, and color mode (Siemens Elegra Ultrasonography Systems, Germany). The far-wall IMT was measured at three different locations on both sides: one measurement from internal carotid artery (ICA), one measurement from bifurcation enlargement (BIF), and three measurements from common carotid artery (CCA) (proximal, middle, and distal from the bifurcation) as reported previously.Citation8,Citation9 The mean CIMT was defined as the mean of right and left ICA, BIF, and the three highest CCA measurements. The reproducibility of the CIMT measurements was examined by conducting another scan 1 week later on eight subjects. In our laboratory, the intra-observer variability is below 10% for CIMT (4.5 ± 3.1%), demonstrating good reproducibility of repeated measurements. The common, internal, and external carotid arteries were also scanned longitudinally and transversely to assess occurrence of plaques. We defined presence of carotid plaque as intima-media thickening that exceeded more than 1.0 mm. IMT was always performed at plaque-free regions.
LABORATORY MEASUREMENTS
Biochemical parameters (creatinine, blood urea nitrogen, glucose, electrolytes, and albumin, hemoglobin, and lipid levels) were measured at the beginning of the study and at 4-week intervals; inflammatory markers [C-reactive protein (CRP), erythrocyte sedimentation rate, white cell count] were determined at the initiation and at 3-month intervals during the 1-year study period. Mean values of biochemical parameters were calculated for each patient at the end of 1 year. Biochemical parameters were measured by means of a computerized autoanalyzer (Hitachi 717; Boehringer-Mannheim, Mannheim, Germany). Total cholesterol (CHO) and triglycerides (TG) were quantified by commercial colorimetric assay method (GPO-PAP and CHOD-PAP; Boehringer-Mannheim). High-density lipoprotein cholesterol (HDL-C) was quantified by the phosphotungstic acid precipitation method. Low-density lipoprotein cholesterol (LDL-C) was calculated by Friedewald formula (LDL-C = CHO-TG/5-HDL-C). Erythrocyte sedimentation rate was determined by the Sedi-system (Becton Dickinson, Paris, France) and CRP was detected by rate nephelometry (IMAGE, Beckman, Brea, California, USA).
STATISTICAL ANALYSIS
All patients who had a baseline and at least one post-randomization measurement were included in the analyses of BP and laboratory variables. Statistical analysis was performed by the Statistical Package for the Social Sciences (SPSS for Windows Software Package; version 15.0). Descriptive statistics were presented as means, medians, and standard deviations. Treatment groups were compared for demographic characteristics and study outcomes using the Student's t-test, Mann–Whitney U-test, Pearson correlation, chi-square, or Fisher's exact test, whichever appropriate. Two-sided hypotheses were evaluated at the statistical significance level of p < 0.05.
RESULTS
Demographics and baseline characteristics
At the beginning, 112 nondiabetic HD patients with hypertension (60 males, 52 females) were included in the study. After titration periods, 20 patients (11 from ramipril and 9 from amlodipine group) were excluded from study because these patients did not reach the target BP despite of the medication with maximum doses of ramipril or amlodipine. The average doses achieved for ramipril and amlodipine groups were 7.67 mg and 7.56 mg, respectively. At the end of the 12 months follow-up, 84 patients (43 patients from ramipril and 41 patients from amlodipine groups) completed the study. In ramipril group, two patients discontinued study (one because of cough, one because of hyperkalemia); however six patients in amlodipine group did not complete protocol (one because of death, one because of hypotension, two because of transplantation, and two because of drug intolerance). Baseline demographic, clinical, laboratory findings were similar between patients in ramipril and amlodipine groups ().
TABLE 1. Baseline characteristics of the study population after titration period (92 chronic HD patients)
Laboratory parameters
During 1-year follow-up, mean eKt/V, interdialytic weight gain, hemoglobin, CRP, erythrocyte sedimentation rate, and lipid levels did not show significant variation and difference between treatment groups ().
TABLE 2. Biochemical parameters (mean ± SD) in ramipril and amlodipine groups during study period (84 study patients completed study)
Blood pressure measurements
Baseline systolic and diastolic BP at study entry was similar in ramipril and amlodipine groups. After 4 weeks of titration period, systolic and diastolic BP reduction and number of patients who reached the target BPs were comparable in both groups. Similarly, during follow-up and end study, mean ambulatory BP measurements included systolic and diastolic and pulse pressure measurements (SBP, DBP, and PP), which did not differ between groups ().
TABLE 3. Blood pressure measurements due to groups during the study period
Echocardiography measurements
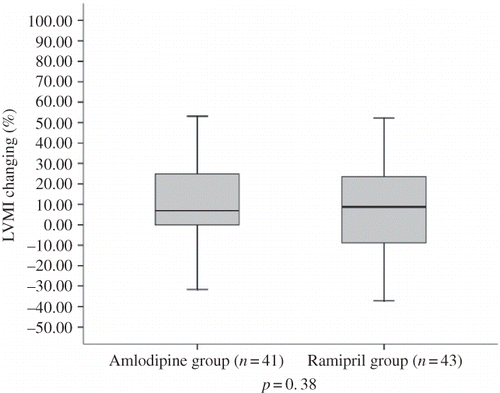
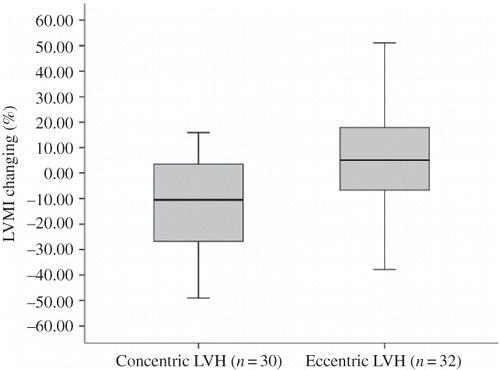
There were no significant differences in LVMI, left ventricular size and wall thickness, and EF between the amlodipine and ramipril groups at entry and at 6 and 12 months after treatment (). During follow-up, LVMI progressed similarly in both treatment groups: 15.2% (95% CI, 4.6–25.7) in amlodipine group, 10.3% (95% CI, 1.10–19.6) in ramipril group (p = 0.38) (). Subgrouping analyses of patients due to the pattern of left ventricular geometry showed that incidence of eccentric type of LVH (n = 32) was similar with concentric type (n = 30). No differences in demographic or clinical parameters were found between the patients with concentric or eccentric LVH at initiation or during 1 year (). Patients with concentric LVH had an average −11.3% (95% CI, −22.4 to −0.17) reduction in LVMI, but subjects with eccentric LVH had an average 4.4% increase (95% CI, −4.4 to 12.8) (p = 0.024) (). We also analyzed the treatment groups due to left ventricular geometry. Eccentric or concentric LVH similarly extended in both groups (). In patients with concentric LVH, LVMI regressed −13.2 % (95% CI, −38.0 to 12.2) in ramipril group (n = 15) and −9.8% (95% CI, −23.3 to 3.6) in amlodipine group (n = 15), but in patients with eccentric LVH, LVMI progressed 3.4% (95% CI, −11.3 to 18.3) in ramipril (n = 17) and 5.2% (95% CI, −5.8 to 16.2) in amlodipine group (n = 15). No significant differences were observed between treatment groups for changing in LVMI in patients with concentric or eccentric LVH.
TABLE 4. LVMI and CIMT measurements during the study period correlated with treatment groups
TABLE 5. Demographic and laboratory parameters (mean ± SD) in patients with concentric and eccentric LVH during study period
When looking for possible associations between the LVMI and variations in other confounding factors such as SBP, DBP, PP, hemoglobin level, iPTH level, and Kt/V that may affect LVMI, baseline LVMI of all patients was directly correlated to SBP (r = 0.37, p = 0.020) and PP (r = 0.43, p = 0.007). Significant relationships were also found between LVMI decreases (ΔLVMI) and ΔSBP (r = 0.80, p = 0.001) and ΔPP (r = 0.75, p = 0.002) decreases in patients with concentric LVH. However, we did not find any correlation between BP measurements and ΔLVMI in patients with eccentric LVH despite comparable reduction in SBP and PP in patients with eccentric or concentric LVH. Similarly, no significant treatment–group interactions were found between change in ΔLVMI and other putative factors.
Carotid ultrasonography measurements
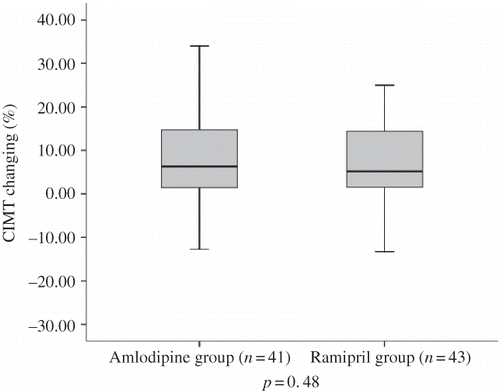
Baseline values for the mean CIMT and the changes in 6 months and 12 months are shown in . After 1 year of treatment, there was a statistically significant increase in CIMT: 0.044 ± 0.079 mm/year in whole patients. The changes in CIMT from baseline in both treatment groups were 8.6 ± 11.0% in the amlodipine group and 6.8 ± 10.9% in the ramipril group (p = 0.48) (). This result indicated no significant differences between the two treatment regimens with respect to changes in CIMT. In the group as a whole, baseline CIMT was directly correlated to PP (r = 0.34, p = 0.036). No significant treatment–group interactions were found between change in mean CIMT and BP measurements (ΔSBP, ΔDBP, ΔPP) and the other confounding factors (causes of renal failure; men and women; smokers and nonsmokers; with plaques and without plaques; those using or not using parenteral iron sucrose, erythropoietin, and statin therapies) (p > 0.05 for all). There appeared to be a significant correlation between progression of LVMI and progression of CIMT (r = 0.38, p = 0.001). The number of patients who had carotid plaques at baseline and at 12 months was not significantly different between groups ().
DISCUSSION
This study showed that in long-term HD patients with hypertension, medication with ramipril 5–10 mg/day or amlodipine 5–10 mg/day has a similar effect on LVMI and CIMT. Impact of antihypertensive treatment on LVMI may be related with the pattern of LVH. Treatment with ramipril or amlodipine may cause regression of LVMI in patients with LVH as concentric pattern by mechanisms added to or independent of BP reduction alone. This decrement in LVH was correlated with the reduction in the SBP and PP. However, BP control by ramipril or amlodipine cannot provide adequate protection to development or progression of eccentric pattern of LVH and atherosclerosis in HD patients.
Echocardiographic studies in HD patients have shown the existence of both concentric and eccentric patterns of LVH. The pathogenesis of left ventricular hypertrophy (LVH) in HD patients is multifactorial and includes hemodynamic factors' overload and such neurohormonal activations.Citation10,Citation11 Eccentric geometric pattern is associated mainly with an increase in hemodynamic preload, whereas concentric hypertrophy seems to be associated mostly with increased hemodynamic afterload. In previous studies of chronic HD patients, concentric LVH was deemed to be the consequence of arterial hypertension, whereas eccentric LVH was considered mostly a result of body fluid overload.Citation12 Anemia is also a major determinant of volume overload and LVH and dilatation in uremic patients.Citation13,Citation14 In our study, no difference was observed in hemoglobin levels, interdialytic weight gain, and dialysis adequacy, and BP control between patients with concentric or eccentric LVH. However, LVH regression was observed in patients with concentric LVH, whereas progression was found in patients with eccentric LVH under antihypertensive treatment. This result may be explained by the findings from previous studies demonstrating that distinct myocyte phenotypes and differential induction of peptide growth factors might activate a distinct form of cardiac muscle cell hypertrophy.Citation15,Citation16 Thus, the same hemodynamic stimulus might lead to different geometric patterns of LVH, depending on individual or genetic factors.
The ability of antihypertensive agents to affect the left ventricular mass has been a topic of discussion. ACE inhibitors have been shown to prevent and reverse hypertension-induced increases in LVMI and collagen deposition in a variety of murine models of hypertension and clinical studies.Citation17–21 The beneficial effect of ACE inhibitors on LVMI is provided by inhibition of the effect of angiotensin II and modulation of sympathetic nervous system activity which neurohumoral and direct tropic factors were implicated in the etiology of LVH.Citation22 Similarly, studies evaluating the effect of CCBs on LVMI showed that dihydropyridine calcium antagonists tend to reduce LVMI when given for a sustained period.Citation23 The decrease in left ventricular mass documented with calcium antagonists is associated with improved ventricular filling and preserved contractility. A calcium channel antagonist led to decrease in myocyte growth and fibroblast activity and tension of ventricular wall, which may contribute to the improvement of LVH.Citation22,Citation23 An increasing number of experimental and clinical reports have analyzed the structural left ventricular changes during ACE inhibition and calcium channel blockage in uremia. The results are controversial. In agreement with our results in ramipril group, Paoletti et al.Citation21 showed that concentric LVH regresses during long-term ACE inhibitor therapy, whereas eccentric LVH remains unaffected despite the similar reduction in BP in HD patients. On the other hand, Wen-Chung Yu et al.Citation23 showed that a 12-month treatment with ramipril did not cause significant regression of LVH in patients on HD. Comparison trials also have had controversial results in uremic patients. London et al. compared the effects of perindopril and a CCB nitrendipine on LVM in patients with ESRD for 1 year. They found perindopril treatment was superior to nitrendipine treatment when induced BP reduction and baseline LVMI were taken into account.Citation21 In contrary, Shibasaki et al.Citation24 compared the effect of antihypertensive agents on LVMI and did not show any difference due to reduction in LVMI between 6 months treatment with enalapril and amlodipine. These findings seem to support our results in patients with concentric LVH. However, these studies did not evaluate the pattern of LVH.
The structures of large arteries are altered in patients with ESRD.Citation25 These changes comprise arterial dilation, intima-media thickening, and increase in stiffness, and are linked to increased risk for cardiovascular disease.Citation26,Citation27 Hypertension may accelerate the progression of atherosclerosis. London et al.Citation28 reported that the chronic increase in systemic blood flow frequently seen in HD patients as a result of the presence of an arteriovenous shunt and volume overload results in an increase in the diameter of the arteries and wall thickness. BP control is of paramount importance in slowing the progression of atherosclerosis and also decreasing the cardiovascular risk in these patients. SBP and PP are predictors of the carotid intima-media thickening in HD patients.Citation29 Adequate BP control should be a major objective in the management of patients with ESRD. A number of randomized trials tested the effect of antihypertensive agents on CIMT progression in non-uremic populations, but yielded conflicting results. A meta-analysis by Wang et al. showed that in the prevention of carotid intima-media thickening, CCBs are more effective than ACE inhibitors, which in turn are more effective than placebo or no treatment.Citation30 This meta-analysis enrolled mainly subjects without uremia. These results cannot be generalized to uremic patients because patients with chronic kidney disease (CKD) are known to have thicker cross-sectional CIMT and accelerated CIMT progression relative to those without CKD. A limited number of experimental and animal studies have shown the effects of antihypertensive agents on atherosclerosis. Kakinuma et al.Citation31 showed the benefit of angiotensin inhibition on vascular lesions such as CIMT and aortic wall of chronic renal failure in subtotally nephrectomized rats. Törnig et al.Citation32 also investigated the effects of ramipril and nifedipine on the thickness of coronary artery and aortic wall in subtotally nephrectomized rats. They had observed that the treatment with ramipril or nifedipine slowed down the progression of vascular wall thickness. They did not found difference between treatment groups. In a clinical trial that enrolled small number of subjects with uremia, vascular protective effects of ramipril were shown in normotensive HD patients.Citation23 In contrary, our analyses showed that thickening of the carotid wall cannot be reversed by antihypertensive therapy despite BP control in HD patients. These results may reflect the characteristics of differences in the study population compared with non-uremic patients. Uremia-related risk factors such as volume overload, inflammation, and oxidative stress may blunt the effect of antihypertensive treatment with ramipril or amlodipine in our study population. Confounding risk factors were similar in both treatment groups. Therefore, we can say that treatments with amlodipine or ramipril have a similar effect on BP and thickening of CIM in this population.
This study contains some limitations in interpreting the data. The trial population was comparably small in number, and the duration of the trial was perhaps short to allow observation of LVM and CIMT changes. A longer period of treatment and/or subjects may be needed to determine their effects on CIMT. In addition, we do not have a control group. Therefore, we were not able to compare drug therapies by amlodipine or ramipril with no treatment in study population.
In conclusion, this study demonstrates that development of atherosclerosis and LVH were continuing processes despite well-controlled BP in nondiabetic HD patients. This may be related with uremia-associated risk factors. Therefore, besides the BP control, confounding risk factors have to be controlled in HD patients. Otherwise, our results confirmed that antihypertensive treatment with ACE inhibitor or CCBs might cause regression of concentric LVH; this type of therapy might not be of benefit in patients with eccentric LVH.Citation21 This finding suggested that assessment of LVH pattern might be useful for predicting cardiovascular prognosis in HD patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hörl MP, Hörl WH. Hemodialysis-associated hypertension: Pathophysiology a therapy. Am J Kidney Dis. 2002;39:227–244.
- Degoulet P, Legrain M, Réach I, Mortality risk factors in patients treated by chronic hemodialysis. Nephron. 1982;31:103–110.
- Huysmans K, Lins RL, Daelemans R, Hypertension and accelerated atherosclerosis in ESRD. J Nephrol. 1998;11(4):185–195.
- Lin YP, Chen CH, Yu WC, Left ventricular mass and hemodynamic overload in normotensive hemodialysis patients. Kidney Int. 2002;62:1828–1838.
- Sehridan D. Regression of left ventricular hypertrophy: Do antihypertensive classes differ? J Hypertens. 2000;18:21–27.
- Stanton AV, Chapman JN, Mayet J, Effects of blood pressure lowering with amlodipine or lisinopril on vascular structure of the common carotid artery. Clinical Sci. 2001;101:455–464.
- Lang RM, Bierig M, Devereux RB, Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Kawagishi T, Nishizawa Y, Konishi T, High resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int. 1995;48:820–826.
- Pascazio L, Bianco F, Giorgini A, Echo color Doppler imaging of carotid vessels in hemodialysis patients: Evidence of high levels of atherosclerotic lesions. Am J Kidney Dis. 2002;28:713–720.
- Weidmann P, Maxwell MH, Lupu AN, Plasma renin activity and blood pressure in terminal renal failure. N Engl J Med. 1971;285:757–762.
- Wang AY, Li PK, Lui SF, Sanderson JE. Angiotensin converting enzyme inhibition for cardiac hypertrophy in patients with end-stage renal disease: What is the evidence? Nephrology (Carlton). 2004;9:190–197.
- Foley RN, Parfrey PS, Harnett JD, The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031.
- Cannella G, Paoletti E, Delfino R, Regression of left ventricular hypertrophy in hypertensive dialyzed uremic patients on long-term antihypertensive therapy. Kidney Int. 1993;44:881–886.
- London GM, Pannier B, Guerin AP, Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol. 2001;12:2759–2767.
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349.
- Verdecchia P, Schillaci G, Borgioni C, Adverse prognostic significance of concentric remodeling of left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995;25:871–878.
- Tan LB, Brilla C, Weber KT. Prevention of structural changes in the heart in hypertension by angiotensin converting enzyme inhibition. J Hypertens. 1992;10(Suppl. 1):31–34.
- Dahlof B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients: A metaanalysis of 109 treatment studies. Am J Hypertens. 1992;5:95–110.
- Wang AY, Li PK, Lui S, Sanderson JE. Angiotensin converting enzyme inhibition for cardiac hypertrophy in patients with end-stage renal disease: What is the evidence? Nephrology 2004;9:190–197.
- London GM, Pannier B, Guerin AP, Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation 1994;90(6):2786–2796.
- Paoletti E, Cassottana P, Bellino D, Left ventricular geometry and adverse cardiovascular events in chronic hemodialysis patients on prolonged therapy with ACE inhibitors. Am J Kidney Dis. 2002;40(4):728–736.
- Ligtenberg G, Blankestijn PJ, Oey PL, Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999;340:1321–1328.
- Yu WC, Lin YP, Lin IF, Effect of ramipril on left ventricular mass in normotensive hemodialysis patients. Am J Kidney Dis. 2006;47(3):478–484.
- Shibasaki Y, Masaki H, Nishiue T, Angiotensin II type 1 receptor antagonist losartan causes regression of left ventricular hypertrophy in end stage renal disease. Nephron 2002;90:256–261.
- London GM, Marchais SJ, Safar ME, Aortic and large artery compliance in end-stage renal failure. Kidney Int. 1990;37:137–142.
- Blacher J, Guerin AP, Pannier B. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001;38:938–942.
- Guerin AP, Blacher J, Pannier B, Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001;103:987–992.
- London GM, Guerin AP, Pannier B, Large artery structure and function in hypertension and end stage renal disease. J Hypertens. 1998;16:1931–1938.
- Shulman NB, Ford CE, Hall WD, Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the Hypertension Detection and Follow-Up Program. The Hypertension Detection and Follow-Up Program Cooperative Group. Hypertension 1989;13(Suppl. 5):80–93.
- Wang JG, Staessen JA, Li Y, Carotid intima-media thickness and antihypertensive treatment: A meta-analysis of randomized controlled trials. Stroke 2006;37:1933–1940.
- Kakinuma Y, Kawamura T, Bills T, Blood pressure-independent effect of angiotensin inhibition on vascular lesions of chronic renal failure. Kidney Int. 1992;42(1):46–55.
- Törnig J, Amann K, Ritz E, Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: The effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol. 1996;7(5):667–675.