Abstract
To date, despite a markedly high incidence of intracerebral hemorrhage (ICH) in patients with end-stage renal disease, only few studies have focused on factors that affect patient's prognosis. To elucidate these factors, we retrospectively investigated 22 consecutive patients who had chronic renal failure, were maintained by hemodialysis (HD), had suffered from ICH, and were hospitalized and treated in our institute from 2006 to 2008. Hematoma volume, blood pressure on admission, blood pressure 3 days after ICH onset, and neurological deterioration significantly affected patient mortality. Progression of neurological symptoms during HD was observed often in patients with hematoma of more than 60 mL or in patients with pontine hemorrhages. Age, gender, duration of HD, anti-platelet or anticoagulant therapies, or maximal dose of nicardipine did not affect patient's prognosis. Based on this study we conclude that controlling blood pressure on admission and within 3 days after onset of ICH may be the most important factor that would improve patient's prognosis. Further, special care might be required for patients with large hematomas (more than 60 mL) or those with brainstem hemorrhages, because progression of neurological symptoms occurs often in such patients.
INTRODUCTION
Cerebrovascular disease is the third most common cause of death in patients who suffer from end-stage renal disease (ESRD) and depend on hemodialysis (HD).Citation1—4 In these patients, the incidence of intracerebral hemorrhage (ICH) has been reported to range from 6.2 to 10.2 per 1000 individuals requiring HD chronically.Citation5—8 The number of patients suffering from ESRD and requiring maintenance HD has been increasing.Citation7 Similarly, the number of patients who are requiring chronic HD and suffer from ICH has been increasing. The mortality in these patients is still high and reportedly ranging from 50% to 90%.Citation5,Citation6,Citation9—11 There have been some reports describing the risk factors and predictors of ICH,Citation2,Citation3,Citation6,Citation8,Citation12—14 but only few have addressed management strategies for improving patient prognosis in such cases.Citation1,Citation7,Citation9,Citation15 To elucidate factors affecting the prognosis of such patients, we retrospectively studied 22 consecutive ESRD patients who were treated in our institute, receiving maintenance HD, and had suffered from ICH.
PATIENTS AND METHODS
Patient population
We retrospectively studied 22 consecutive patients receiving maintenance HD for ESRD, who were admitted to our hospital for treatment of ICH between January 2006 and December 2008. The characteristics of these patients are summarized in .
TABLE 1. Characteristics of patients
Management of patients
In order to maintain patient's systolic blood pressure (sBP) below 160 mmHg, continuous intravenous antihypertensive treatment with nicardipine was initiated as soon as ICH was diagnosed. In addition, an antifibrinolytic agent was administered intravenously. HD was continued as prescribed before admission; however, patients receiving heparin were switched to nafamostat mesylate after admission. Osmotic diuretics such as glycerol were also administered during HD to reduce cerebral edema. HD was not performed on the day of onset of ICH. In our series, no patient received craniotomy for treatment. In our cases, evacuation of hematoma by craniotomy was considered only in patients with transtentorial herniation and if the patients' families opted for surgery. One patient received stereotactic drainage of hematoma and another received external ventricular drainage for acute obstructive hydrocephalus.
Analysis of the factors affecting patient outcome
The interrelationships between the following factors and patient outcome were analyzed: age, gender, basic renal disease, duration of HD to the onset of ICH, use of antiplatelet agents, use of anticoagulants, volume of hematoma, location of hematoma, sBP on admission, sBP 3 days after onset of ICH, maximum doses of medication for sBP control, consciousness level on admission, convulsion, and progression of neurological symptoms during HD. The factors affecting hematoma enlargement were also analyzed.
Statistical analysis
Statistical analyses were performed using the Statview software (Abacus Corp., Cary, North Carolina, USA) and GraphPad PRISM 4 (GraphPad Software, Inc., La Jolla, California, USA). Variables found to be significant were used in a logistic regression model. Because our case numbers in this series may have been too small to be analyzed by multivariate analyses, we also tested the relationships affecting these variables and mortality, outcome, and enlargement of hematoma using univariate analyses (Mann–Whitney U-test). Differences between groups were assessed by analysis of variance (ANOVA) and the Student's t-test. Data were expressed as the mean ± standard deviation. A p-value of <0.05 was considered statistically significant.
RESULTS
Overall factors that affect patient outcome
The results of logistic analyses of factors that affect patient's mortality are shown in . Volume of hematoma, sBP on admission, and sBP 3 days after onset of ICH were identified as significant predictors. In the univariate analysis, the following were also identified as significant factors: consciousness level on admission (p = 0.0002) and progression of neurological symptoms during dialysis (p = 0.04). shows the correlation of hematoma volume and outcome.
TABLE 2. Factors that affect the mortality of patients
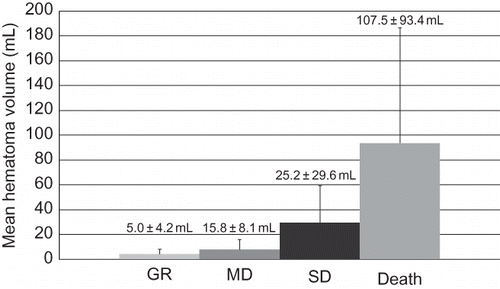
FIGURE 1. Hematoma volume on admission and outcome (Glasgow outcome scale). GR, good recovery; MD, moderately disabled; SD, severely disabled. GR group: 3 cases, volume of hematoma was 5.0 ± 4.2 mL; MD group: 5 cases, volume of hematoma was 15.8 ± 8.1 mL; SD group: 6 cases, volume of hematoma was 25.2 ± 29.6 mL; Deceased group: 8 cases, volume of hematoma was 107.5 ± 93.4 mL. There was a significant difference between GR and Death (p = 0.029).
Factors affecting enlargement of hematoma
In our analyses, sBP on admission, sBP 3 days after admission, the maximum dose of nicardipine used for sBP control, and use of antiplatelet agents and/or anticoagulants did not affect enlargement of hematoma in patients.
Factors affecting neurological deterioration during HD
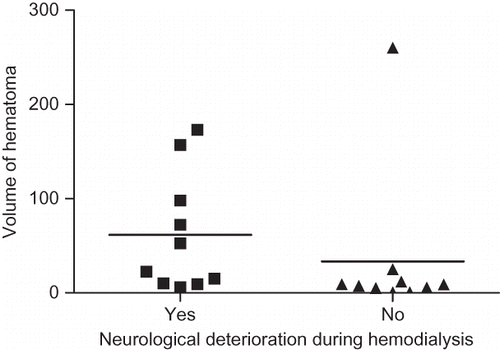
Patients with large hematoma volume showed significant deterioration of neurological symptoms during HD (p = 0.03), especially those patients with hematoma volumes exceeding 60 mL (). Furthermore, location of hematoma also correlated significantly with deterioration of patient's neurological symptoms during dialysis (p = 0.01). Among them, deterioration of neurological symptoms occurred more often in patients with pontine hematomas than with other hematomas. In our study, all patients with pontine hematoma showed deterioration of neurological symptoms during HD.
FIGURE 2. Volume of hematoma on admission and neurological deteriorations during hemodialysis. Volume of hematoma in the group with neurological deterioration (11 cases) was 61.58 ± 62.57 mL. Volume of hematoma in the group without neurological deterioration (11 cases) was 33.37 ± 79.93 mL. There was a significant difference between these groups (p = 0.028).
DISCUSSION
In this study, the following factors significantly affected the mortality of HD patients with ICH: volume of hematoma on admission, sBP on admission, sBP 3 days after the onset of ICH, and neurological deterioration during HD. Moreover, neurological deterioration during HD was especially observed in patients with pontine hemorrhages and in patients with hematomas exceeding 60 mL in volume. We used multivariate and univariate analyses because we were concerned that the case numbers in this series may not be large enough to be analyzed by multivariate analyses, and that evaluation only by multivariate analyses may not have been accurate.
The prognosis of ICH concurrent with chronic HD has a dismal outcome, with overall mortality rate reported to be between 43.8% and 83%.Citation5,Citation6,Citation9—11 However, in this study, the mortality rate in such cases was found to be 36.5%, which was better than that in the previously reported cases. This may be attributable to the following factors: our series involved a smaller number of patients and the hematoma volume on admission was smaller in our series than in the reported cases. However, in our series, the number of patients with brainstem hemorrhages was larger than that in the previous reports; therefore, factors that caused a good prognosis in our cases may not be simply attributed to the volume of hematoma.
There have been some reports regarding predictors affecting the onset of ICH in the chronic HD patients.Citation2,Citation3,Citation6,Citation8,Citation12—14 However, to our knowledge, there have been few reports discussing predictors that affect the prognosis of ICH in chronic HD patients.Citation1,Citation7,Citation9,Citation15 In our study, similar to the findings reported previously, blood pressure after onset of ICH was one of the most important factors improving patient prognosis.Citation16,Citation17 However, in our study, we showed that sBP 3 days after ICH onset was also the important factor of patient outcome. On the basis of our findings, strict sBP control after onset of ICH seems to be the most important factor to prevent hematoma progression and improve prognosis. However, others believe that elevation of blood pressure occurs merely as a result of the severity of hematoma. In order to prove these conflicting theories, an interventional trial will be required in the future.
In our study, we used nicardipine instead of diltiazem to control sBP. The effect of nicardipine is usually rapid, its half-life is short, and its blood concentration can be easily controlled.Citation18 Furthermore, nicardipine does not have the serious cardiovascular side effects that diltiazem has, such as cardiac blockades.Citation19 In HD patients with ICH, large doses of hypertensive agents are sometimes needed to maintain adequate sBP level. However, if diltiazem were to be used for sBP control in such patients, such high doses of diltiazem would be necessary that would induce cardiac complications. Therefore, the use of diltiazem should be avoided in such cases. For these reasons, we recommend the use of nicardipine for sBP control. In Japan, use of nicardipine has been reportedly linked with possible ICH progression, but in our study, there were no cases of hematoma progression caused by nicardipine administration.
Neurological deterioration during HD is also an important factor that affected prognosis in our study and could be attributed to HD disequilibrium syndrome. To avoid this syndrome, some previous reports recommended the use of continuous hemofiltration (CHF) in the acute phase of ICH.Citation9 On the contrary, Miyahara et al. recommended HD with nafamostat mesylate except on day 1.Citation7 In this study, similar to Miyahara et al., we continued HD using nafamostat mesylate on day 2 or 3 after onset of ICH and maintained tertiary a week. Our reasons for continuing HD instead of CHF even in the acute phase of ICH are as follows: (1) CHF application in all patients in a local hospital may be limited by manpower and equipment availability and (2) although nafamostat is reported to improve prognosis of ICH in HD patients,Citation20 using CHF would also require continuous anticoagulant infusion and, as a result, some bleeding may occur even if nafamostat mesylate were to be used. It was reported that disequilibrium syndrome was prevented by administration of glycerol during HD.Citation15 However, this has not been proven solidly yet. In this study, we administered glycerol routinely during HD but were unable to completely prevent neurological deterioration during HD. In our study, neurological deterioration during HD was often observed in patients with hematomas of large volumes and brainstem hemorrhages. Especially in patients with brainstem hemorrhages, serious neurological deterioration, such as decrease in consciousness or respiratory disturbances, was often observed. Important vital nervous centers, such as the ascending reticular activation system (ARAS), the respiratory center, and the vasomotor center, are tightly clustered in the brainstem. Thus, if edema around a hematoma progressed to the brainstem, these important systems could be easily affected, resulting in life-threatening neurological deterioration during HD. Therefore, to improve the prognosis for patients with brainstem hematomas, preparation for sudden respiratory or vasomotor deterioration during HD is necessary. Similarly, in patients with large hematomas, surgical treatment for intracranial hypertension without craniotomy, such as drainage of hematoma or the cerebrospinal fluid, may be useful in preventing neurological deterioration during HD.
CONCLUSION
We retrospectively analyzed the predicting factors that affect prognosis in chronic HD patients with ICH. To improve the prognosis of such patients, strict control of blood pressure and adequate preparation for HD disequilibrium syndrome are indispensible.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Ikeda K, Tsuchimochi H, Takeno Y, Clinical analysis of the patients with hemodialysis associated with intracerebral hematoma. No Shinkei Geka. 2004;32:1133–1137.
- Iseki K, Fukiyama K. Predictors of stroke in patients receiving chronic hemodialysis. Kidney Int. 1996;50:1672–1675.
- Iseki K, Kawazoe N, Osawa A, Survival analysis of dialysis patients in Okinawa, Japan (1971–1990). Kidney Int. 1993;43:404–409.
- Japanese Society for Dialysis Therapy. An overview of regular dialysis treatment in Japan. Tokyo, Japan: Japanese Society for Dialysis Therapy, 2001, pp 78–691.
- Iseki K, Tozawa M, Iseki C, Clinical demographics and long-term prognosis after stroke in patients on chronic hemodialysis. Nephrol Dial Transplant. 2000;15:1808–1813.
- Kawamura M, Fujimoto S, Hisanaga S, Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31:991–996.
- Miyahara K, Murata H, Abe H. Predictors of intracranial hematoma enlargement in patients undergoing hemodialysis. Neurol Med Chir (Tokyo). 2007;47:47–52.
- Onoyama K, Ibayashi S, Nanishi F, Cerebral hemorrhage in patients on maintenance hemodialysis. CT analysis of 25 cases. Eur Neurol. 1987;26:171–175.
- Murakami M, Hamasaki T, Kimura S, Clinical features and management of intracranial hemorrhage in patients undergoing maintenance dialysis therapy. Neurol Med Chir (Tokyo). 2004;44:225–233.
- Noda T, Suzuki S, Miyazaki S, Cerebrovascular disease in chronic hemodialysis patients. J Jpn Soc Dial Ther. 2000;33:1389–1400.
- Onoyama K, Kumagai H, Miishima T, Incidence of strokes and its prognosis in patients on maintenance hemodialysis. Jpn Heart J. 1986; 27:685–691.
- Seliger SL, Gillen DL, Longstreth WT, Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609.
- Seliger SL, Gillen DL, Tirschwell D, Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol. 2003;14:2623–2631.
- Toyoda K, Fujii K, Fujimi S, Stroke in patients on maintenance hemodialysis: A 22-year single-center study. Am J Kidney Dis. 2005;45:1058–1066.
- Ikeda K, Tsuchimochi H, Fukushima T. Intracerebral hemorrhage of hemodialysis patients. Nippon Rinsho. 2006;64:542–545.
- Anderson CS, Huang Y, Wang JG, INTERACT Investigators: Intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT): A randomized pilot trial. Lancet Neurol. 2008;7:391–399.
- Anderson CS, Huang Y, Arima H, INTERACT Investigators: Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: The Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT). Stroke. 2010;41:307–312.
- Iliopoulou A, Turner P, Warrington SJ. Acute hemodynamic effects of a new calcium antagonist, nicardipine, in man. A comparison with nifedipine. Br J Clin Pharmacol. 1983;15:59–65.
- Smith MS, Verghese CP, Shand DG, Pritchett EL. Pharmacokinetic and pharmacodynamic effects of diltiazem. Am J Cardiol. 1983;51:1369–1374.
- Yang JW, Han BG, Kim BR, Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage. Renal Failure. 2009;31:668–675.