Abstract
Rational: Peritoneal sclerosis is one of the important complications of long-term peritoneal dialysis (PD). In this study, efficacy of atorvastatin on peritoneal histology and functions in non-uremic rats on PD was tested. Objectives: Twenty-two non-uremic Wistar albino rats were randomized into three groups: Sham (intraperitoneal saline), peritoneal dialysis (PD, intraperitoneal 3.86% dextrose containing PD solution), and treatment (TX, intraperitoneal 3.86% dextrose containing PD solution plus atorvastatin added into drinking water). At the end of a 4-week period, 1 h peritoneal equilibration test was performed. Serum lipids and certain cytokines, mediators, markers, and antioxidant enzyme activities in serum and dialysate were studied. Peritoneal thickness was measured and peritoneal inflammation, fibrosis, and vascular proliferation were scored in histological sections. Main findings: In histological examinations, inflammation, fibrosis, and vascular proliferation were significantly more frequent in PD group than Sham group and it seemed to decrease significantly when atorvastatin was used in conjunction with PD. Additionally, peritoneum was significantly thicker in PD group when compared to that of Sham and TX groups. Serum parameters did not significantly differ between groups. On the other hand, dialysate glutathione reductase (GR) activity and TGF-β were significantly lower in TX group than that of the PD group, whereas dialysate IL-6 level was higher in TX group. Principal conclusions: In our study, atorvastatin use appeared to diminish structural changes in peritoneum. Decreased expression of TGF-β in dialysate may be one of the possible underlying mechanisms.
INTRODUCTION
Peritoneal sclerosis is one of the important complications of long-term peritoneal dialysis (PD) treatment resulting in ultrafiltration failure and in turn withdrawal of the treatment. Its prevalence, histological findings, and symptoms differ. Varying degrees of peritoneal sclerosis is almost always noted in long-term PD patients.Citation1–3
Bioincompatible PD solutions may impair the structure and function of the peritoneum, which may cause ultimately peritoneal sclerosis and ultrafiltration failure. Besides PD components, uremic toxicity may also contribute to this injury. Morphologically mesothelial denudation, interstitial fibrosis, neovascularization, and vascular alterations are noted. Although the mechanism(s) underlying these alterations is (are) not completely known, cytokines and growth factors released by mesothelial cells and macrophages are proposed in the pathogenesis.Citation1–8
To eliminate the deleterious effects of long-term PD on peritoneal function and structure, many agents including valsartan, lisinopril, enalapril, and octreotide were tried yielding some beneficial outcomes.Citation9–11
Statins are generally prescribed to treat hyperlipidemia; however, data concerning anti-inflammatory, antiproliferative, and antioxidant effects of statins are accumulating.Citation12–16 In this study, we aimed to test the effects of atorvastatin on peritoneal functions and peritoneal histology in rats on PD in terms of the pathogenesis.
MATERIAL AND METHODS
Twenty-two non-uremic Wistar albino rats weighing 200–250 g were enrolled into the study. Rats were housed in polycarbonate cages in a room maintained at a constant temperature (25°C) with 12 h light–dark cycle. All rats were given unlimited access to standard rat chow and water. The animals were randomized into three groups: Sham (n = 7), peritoneal dialysis (PD, n = 7), and treatment (TX, n = 8) groups.
Sham group received daily intraperitoneal injection of 10 mL saline, PD and TX groups received daily intraperitoneal injection of 10 mL of 3.86% dextrose containing PD solution (Dianeal, Healthcare Corporation Baxter, Chicago, Illinois, USA) for 4 weeks. TX group were additionally given atorvastatin calcium (Sanovel Drug Industry, Istanbul, Turkey) added into drinking water at a dose of 80 mg/mL.Citation17
At the end of 4 weeks, 1 h peritoneal equilibration test (PET) was performed; 20 mL 2.27% dextrose containing PD solution at 37°C was introduced into peritoneal cavity by means of a 22 gauge needle syringe. After the injection, rats were allowed to mobilize freely. At the end of 1 h, under ether anesthesia, intra-abdominal fluid was aspirated through a midline incision and its volume was noted. After blood samples were drawn from the heart, peritoneal samples were taken far from the injection site. After sample collection, rats were killed by hypovolemia.
Histopathological examination
Tissue samples from sites of most severe adhesions depending on gross examination were taken from abdominal wall and liver surface for histological examination and were immediately fixed in formalin.
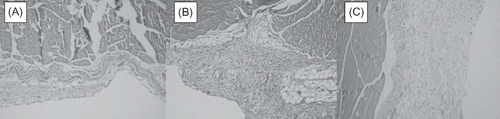
Macroscopically, tissues from the abdominal wall were sectioned vertically with 3 mm in thickness. After tissue processing, they were embedded in paraffin and sections were stained with H&E and modified Masson's Trichrome (MT). Light microscopic examination revealed inflammation and fibrosis as well as vascular proliferation of different quantities at the parietal peritoneum of the abdominal wall. The inflammation and fibrosis were scored semi-quantitatively through the H&E- and MT-stained slides, respectively, as follows: 0: none, 1: mild, 2: moderate, 3: severe. Also vascular proliferation was semi-quantitatively scored for the peritoneum as follows: 0: none, 1: mild, 2: moderate, 3: severe.Citation18
Digital images from selected areas were captured through a light microscope at 40× magnification for the liver capsule and 20× for peritoneum (Labophot-2; Nikon, Tokyo, Japan) with a digital 3 CCD video camera (Olympus DP70, Olympus Optical Co., Ltd., Tokyo, Japan), connected to a light microscope (Olympus BX51, Olympus Optical Co., Ltd.). The images were processed by image analysis software (Bs200D Image Analysis Software, BAB Mühendislik, Ankara, Turkey) and saved on a computer. From each section of the peritoneal wall, 10 measurements were performed for the thickness of the peritoneum and the mean of these were used for statistical analysis.Citation19,Citation20
Biochemical analysis
Urea, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, interleukin1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), total glutathione (GSH), malonyldialdehyde (MDA), nitric oxide (NO), and catalase (CAT) were studied in serum samples.
Urea, glucose, protein, IL-1β, IL-6, TGF-β, glutathione peroxidase (GPX), glutathione reductase (GR), MDA, and NO were studied in dialysate samples. Additionally, ultrafiltration volume was noted and dialysate/plasma urea (D/P urea) and Dialysate1/Dialysate0 glucose (D1/D0 glucose) were calculated in 1 h PET.
Urea, total cholesterol, HDL cholesterol, LDL cholesterol, glucose were measured by commercial kits in an autoanalyzer (Abbott, C8000 autoanalyzer, Abbott Park, Illinois, USA). Protein determination was done by Lowry's method.Citation21
Determination of IL-1β
IL-1β levels were determined via commercial rat ELISA kit (IBL Co., Ltd., Gunma, Japan); catalog number was 27193. The test results were calculated by bioelisa reader Elx800 using standard curve.
Determination of IL-6
IL-6 levels were determined via commercial rat ELISA kit (IBL Co., Ltd.); catalog and lot numbers were 17,194 and OL-527. The test results were calculated by bioelisa reader Elx800 using standard curve.
Determination of TNF-α
TNF-α levels were determined via commercial rat ELISA kit (IBL Co., Ltd.), catalog number was 17,194. The test results were calculated by bioelisa reader Elx800 using standard curve.
Determination of TGF-β1
TGF-β1 levels were determined via commercial ELISA kit (BioSource Int, Inc., Camarillo, California, USA); catalog and lot numbers were KAC1688 and 043503, respectively. Test results were calculated by bioelisa reader Elx800 using standard curve.
Determination of total GSH
Total GSH measurements were performed by the method of Tietze.Citation22 In brief, 0.5 mL sample or standard solution was mixed with 0.25 mL of 1 mol/L sodium phosphate buffer (pH 6.8) and 0.5 mL 5–5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 0.8 g/L in the phosphate buffer) for 5 min. Then, the absorbance was measured at 412 nm using a Shimadzu UV-160 spectrophotometer. The GSH concentration was determined using standard aqueous solutions of GSH. Results were expressed as milligrams per deciliter.
Determination of MDA (TBARS)
The MDA production and hence lipid peroxidation was assessed in the tissues by the method of Ohkowa.Citation23 MDA forms a colored complex in the presence of TBA, which is detectable by measurement of absorbance at 532 nm. Absorbance was measured with Shimadzu UV-160 spectrophotometer. 1,1′,3,3′-Tetraethoxypropane was used as a standard and the results were expressed as nanomoles per milliliter.
Determination of nitric oxide
NO was assayed by a modification of cadmium-reduction method as mentioned by Navarro-Gonzalves.Citation24 The nitrite produced was determined by diazotization of sulphanylamide and coupling to naphthylethylene diamine. For the measurement of NO, 400 μL sample was denatured by adding 80 μL 30% ZnSO4 solution, stirring, and then centrifuging at 10,000g for 20 min at 4°C. First, we activated Cd granules using CuSO4 solution in glycine-NaOH buffer. Then 100 μL of deproteinized samples and standards was added. This reaction uses pre-treatment of samples to reduce nitrate to nitrite, which can be accomplished by catalytic reactions using enzyme or Cd. The samples were analyzed spectrophotometrically using a microplate reader and quantified automatically against KNO3 standard curve and the results were expressed as micromoles per liter.
Catalase activity measurement
Catalase (CAT) activity measurement in erythrocyte lysate was measured by the method of Aebi. The reaction mixture was 50 mM phosphate buffer (pH 7.0), 10 mM H2O2, and erythrocyte lysate. The reduction rate of H2O2 was followed at 240 nm for 30 s at room temperature. Catalase activity was expressed in units per gram Hb.Citation25
Glutathione peroxidase activity measurement
We determined the glutathione peroxidase (GPX) activity by a minor modification of the method of Paglia and Valentine. Ten microliters of sample was transferred to a 500 mL quartz cuvette, containing 950 μL of the reaction mixture (Tris buffer, 50 mmol/L, pH 7.6, containing per liter, 1 mmol of Na2EDTA, 2 mmol of reduced glutathione, 0.2 mmol NADPH, 4 mmol sodium azide, and 1000 U of GR). Mixture was incubated at 37°C for 5 min. Then the reaction was initiated by adding 25 μL of H2O2, 8.8 mmol/L (% 30), and the decrease in NADPH absorbance at 340 nm was followed for 3 min. The nonenzymatic reaction rate (blank) was determined by substituting water for the supernatant and recording the decrease in NADPH absorbance.
Glutathione reductase activity measurement
Glutathione reductase (GR) was assayed following the oxidation of NADPH at 340 nm at 37°C. The reaction was initiated by the addition of 50 μL supernatant to 1 mL of assay mixture containing 50 mmol/L Tris, pH 7.6, 100 μmol/L Na2EDTA, 4 mmol/L GSSG, 120 μmol/L NADPH. A blank cuvette was prepared in which the sample was replaced with water. The reaction was linear for 2–3 min.Citation26
Statistics
All data were presented as mean±SEM. SPSS software was used for statistical analysis. Because of limited number of rats in each group, nonparametric tests were applied. Kruskal–Wallis test was used for overall comparison of all three groups with each other. In case Kruskal–Wallis test yielded significant difference, Mann–Whitney U-test was applied to identify the origin of the difference. For crosstab analysis, Fisher's exact chi-square test was used. p < 0.05 was accepted as significance level.
The study protocol was approved by the Local Ethical Committee of Laboratory Animals at Dokuz Eylul University Hospital at Izmir-Turkey.
RESULTS
All rats completed the study. Body weights of the rats were similar in Sham (225.57 ± 3.90 g), PD (224 ± 7.68 g), and TX (235.25 ± 7.18 g) groups ().
Table 1. Comparison of peritoneal equilibration tests and dialysate levels parameters between groups
Concerning 1 h PET and basic dialysate parameters, ultrafiltration volumes and dialysate protein loss were similar in all groups. Dialysate/plasma (D/P) urea ratio was similar in all groups whereas D1/D0 glucose ratio differed significantly among all three groups, which appeared to result from the differences between Sham–PD and Sham–TX comparisons. It was significantly higher in Sham (0.70 ± 0.05) group than that of the PD (0.43 ± 0.08) and TX (0.56 ± 0.04) groups. It was statistically similar in TX and PD groups ().
Dialysate IL-1β, TNF-α, GPx, MDA, and NO were similar in all three groups. However, dialysate IL-6, TGF-β, and GR were significantly different from each other ().
Dialysate IL-6 levels were statistically different among groups. Significantly higher dialysate IL-6 levels in TX (304.16 ± 42.86 pg/mL) group compared to that of Sham (237.14 ± 15.07 pg/mL) and PD (238.33 ± 5.00 pg/mL) groups was the cause of the difference.
Dialysate TGF-β differed significantly among groups and it was because of lower dialysate TGF-β levels in TX (162.88 ± 32.26 pg/mL) group than that of the PD (391.83 ± 92.41 pg/mL) group.
Dialysate GR activity was significantly lower in TX group (1.71 ± 0.19 mU/mL) than that of the Sham (4.82 ± 2.11 mU/mL) and PD (2.84 ± 0.33 mU/mL) groups. It was similar in Sham and PD groups. Results of dialysate parameters are outlined in .
Considering all the serum parameters, no significant difference was observed. Results of serum parameters are presented in .
Table 2. Serum parameters
As the number of rats in each histological score groups was limited to perform crosstab analysis, inflammation, fibrosis, and vascular proliferation scores were re-defined as “Absent” (none) or “Present” (mild, moderate, or severe involvement).
When histological sections were considered, inflammation, fibrosis, and vascular proliferation were significantly more frequently observed in PD (100%, 100%, 71.4%, respectively) group when compared to that of the Sham (0%, 14.3%, 0%, respectively) group and it seemed to significantly decrease when atorvastatin was used in conjunction with PD (i.e., TX group 37.5%, 37.5%, 12.5%, respectively) (). When groups are compared with each other separately, Sham–PD and PD––TX comparisons yielded significant results, whereas Sham and TX groups were similar with respect to histology. Additionally, peritoneum was significantly thicker in PD (152.28 ± 19.07) group when compared to that of the Sham (49.57 ± 10.67) and TX (46.5 ± 9.72) groups, and the peritoneal thickness was similar in Sham and TX groups ( and ).
Table 3. Histological evaluation
DISCUSSION
One of the limitations of the PD is progressive decrease in dialysis adequacy and ultrafiltration failure because of peritoneal fibrosis which may frequently give rise to withdrawal of the PD.Citation27–31 Prevalence of peritoneal fibrosis increases by time on PD which is reported to be 3% and 31% in the first and sixth years of the treatment, respectively.Citation4
Peritoneal changes in long-term PD patients result from exposure to nonphysiological PD solutions and recurrent bacterial peritonitis episodes.Citation32,Citation33 It was shown in an animal model that glucose exposure was more deleterious to peritoneum than low pH, lactate, and hyperosmolality.Citation34 Because glucose traverses mesothelium readily, all parts of mesothelium face extremely high concentrations of glucose, overshooting the plasma glucose level of diabetics explaining development of diabetiform changes in microvasculature characterized by neoangiogenesis.Citation11,Citation33,Citation35,Citation36 Morphological changes in peritoneum during CAPD are in two main forms. First, thickness of submesothelial compact layer increases owing to deposition of collagen and second, diabetiform changes in vascular structure such as increased number of capillaries, smooth muscle hyperplasia, subendothelial thickening, and collagen deposition.Citation19,Citation27,Citation37–40
Although mechanisms inducing peritoneal fibrosis at tissue level are not known clearly, interstitial fibrosis is the common pathway. Initial stages of fibrosis are usually related to induction of inflammation operated by activated macrophagesCitation41 which release cytokines and growth factors followed by increased extracellular matrix (ECM) turnover, collagen deposition, and fibrosis.Citation37 Although many cytokines such as TGF-β, connective tissue growth factor (CTGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), IL-6, and IL-1β may have roles in the pathogenesis of peritoneal fibrosis,Citation42–46 main mediator is TGF-β, and TGF-β1 isoform contributes primarily.Citation47 Failure of TGF-β1 levels to decrease is a constant feature of the fibrotic diseases.Citation36,Citation41 Underlying factor preventing TGF-β1 to decrease is unknown. Recurrent and continuing noxious stimuli may be the cause.Citation18,Citation41 A number of medications, including valsartan, lisinopril, enalapril, octreotide, dipyramidole, and pentoxyphyline, were used to preserve longevity of PD yielding some degree of success.Citation9–141,Citation37,Citation48 There is only one report concerning the effects of atorvastatin.Citation49
In this study, atorvastatin was chosen to test its effects on the development of peritoneal fibrosis and to consider possible pathogenetic mechanisms using the aforementioned experimental model,Citation48 because of antioxidant, anti-inflammatory,Citation50–52 antiproliferative, atherosclerotic plaque stabilizing, anticoagulant, and renoprotectiveCitation53–55 effects of statins, independent of their lipid-lowering effects.Citation46
Statins are known to inhibit modification of various proteins involved in signal transduction. Such unmodified proteins cannot attach to the cell membrane preventing signal transduction from cell surface to the cell nucleus. This is applicable in many situations including the Ras proteins which are implicated in non-smad pathways of TGF-b1 signaling.Citation56
Non-infectious inflammation of peritoneum in PD secondary to chemical and physical irritation is shown to be related to the serofibrinous exudation in peritoneum. Statins prevent peritoneal fibrin deposition and development of adhesions by increasing peritoneal fibrinolytic activity by increasing t-PA and decreasing plasminogen activator inhibitor-1 production.Citation57
TGF-β is the major activator of ECM synthesis and gives rise to fibrosis. Statins were found to decrease TGF-β mRNA expression and shown to be of help in glomerulosclerosisCitation58 and in cyclosporine nephrotoxicity.Citation53 Additionally, atorvastatin use was shown to be beneficial in prevention of renal inflammation and fibrosis in an experimental model of chronic allograft nephropathy.Citation50
In our study, mild inflammation, fibrosis, and vascular proliferation were more prevalent and peritoneum was thicker in PD group than Sham group. The experimental model that is used in this study was able to induce deleterious effects on peritoneum. Although histology differed among groups, ultrafiltration volumes, protein loss in dialysate, D/P urea were similar in all groups; on the other hand, D1/D0 glucose ratio was significantly higher in Sham group than the others. Decreased D1/D0 glucose ratio in PD group may reflect high permeable status owing to early functional derangements owing to inflammation. When the effects of atorvastatin on peritoneal structure were evaluated, inflammation, fibrosis, and vascular proliferation were noted to be significantly less frequent and peritoneum was significantly thinner when atorvastatin was used in conjunction with experimental PD model. Partial restoration of the high glucose transporter state by use of atorvastatin may point out the beneficial effects of atorvastatin. Additionally, histological parameters were statistically similar in Sham and TX groups. In other words, atorvastatin use diminished deleterious effects of hypertonic glucose-containing solutions. The data point out the beneficial effects of atorvastatin. In a previous publication by Duman et al., beneficial effects of atorvastatin on peritoneal sclerosis were reported; they documented decreased dialysate TGF-β and VEGF levels by use of atorvastatin and put forward that favoring effects of atorvastatin were because of its anti-inflammatory effects.Citation49 However, aforementioned article lacks insight into the pathogenesis. In our study, pathogenetic processes were assessed through pro-inflammatory cytokines and markers of the antioxidant system, even if no significant change was observed.
When serum markers were considered, serum total cholesterol and LDL cholesterol levels did not present significant changes expected by atorvastatin use; it may be because of limited number of rats enrolled. However, significantly lower serum total cholesterol level was observed by use of atorvastatin in the study by Duman et al.Citation49 In our study, levels of aforementioned serum cytokines and antioxidant–oxidant system markers were similar in all three groups. Dialysate IL-6 level was significantly higher in TX group than that of the Sham and PD groups. On the other hand, dialysate GR activity was lower in TX group than the rest which fails to support antioxidant and anti-inflammatory mechanisms suggested. In several experimental models, statins inhibited NF-κB activation, which is a pivotal transcription factor regulating the expression of a variety of inflammatory genes.Citation59 The inhibition of NF-κB activation could explain the reduced synthesis of inflammatory cytokines, such as interleukin-6, under these in vitro conditions.Citation60 It may also be possible that higher levels of TGF-β in the PD group are responsible, because TGF-β1 is one of the most potent anti-inflammatory cytokine.Citation61
Low number of subjects, lack of quantification of the drug ingested by each rat, and use of drug in higher dose in comparison to clinical practice are the main limitations of the study. Additionally, because uremia gives rise to many alterations in oxidative and inflammatory status,Citation62,Citation63 use of non-uremic rats instead of uremic ones may not reflect exactly what happens in PD patients.
In our study, atorvastatin use in conjunction with PD has resulted in improved histology and partial restoration of the high permeable status pointing out its beneficial effects. Combining the data, antioxidant and anti-inflammatory mechanisms do not appear to be operating mechanisms. Confirming the data of Duman et al.,Citation49 decreased expression of TGF-β in dialysate may be one of the possible underlying mechanisms.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Jimenez-Heffernan JA, Aguilera A, Aroeira LS, Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch. 2004;444:247–256.
- Nishino T, Miyazaki M, Abe K, Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int. 2003;64(3):887–896.
- Günal AI, Duman S, Sen S, By reducing TGFß1, octreotide lessens the peritoneal derangements induced by a high glucose solution. J Nephrol. 2001;14:184–189.
- Selgas R, Fernandez-Reyes MJ, Bosque E, Functional longevity of the human peritoneum: How long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am J Kidney Dis. 1994;23(1):64–73.
- Di Paolo N, Garosi G. Peritoneal sclerosis. J Nephrol. 1999;12(6):347–361.
- Plum J, Hermann S, Fusshöller A, Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int Suppl. 2001;78: S42–S47.
- Honda K, Nitta K, Horita S, Yumura W, Nihei H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron. 1996;72(2): 171–176.
- Rubin J, Herrera GA, Collins D. An autopsy study of the peritoneal cavity from patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1991;18(1):97–102.
- Duman S, Sen S, Duman C, Oreopoulos DG. Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int J Artif Organs. 2005;28(2):156–163.
- Duman S, Wieczorowska-Tobis K, Styszynski A, Kwiatkowska B, Breborowicz A, Oreopoulos DG. Intraperitoneal enalapril ameliorates morphologic changes induced by hypertonic peritoneal dialysis solutions in rat peritoneum. Adv Perit Dial. 2004;20:31–36.
- Gunal AI, Celiker H, Akpolat N, Ustundag B, Duman S, Akcicek F. By reducing production of vascular endothelial growth factor octreotide improves the peritoneal vascular alterations induced by hypertonic peritoneal dialysis solution. Perit Dial Int. 2002;22(3):301–306.
- Oda H, Keane WF. Recent advances in statins and the kidney. Kidney Int Suppl. 1999;71:S2–S5.
- Schonbeck U, Libby P. CD40 signalling and plaque instability. Circ Res. 2001;89:1092–1103.
- Rabbani R, Topol EJ. Strategies to achieve coronary arterial plaque stabilization. Cardiovasc Res. 1999;41:402–417.
- Koh KK. Effects of statins vascular wall: Vasomotor function, inflammation and plaque stability. Cardiovasc Res. 2000;47:648–657.
- Yoshino G, Hirano T, Kazumi T. Fluvastatin increases LDL particle size and reduces oxidative stress in patients with hyperlipidemia. J Atheroscler Thromb. 2003;10(6):343–347.
- Marumo H, Satoh K, Yamamoto A, Kaneta S, Ichihara K. Simvastatin and atorvastatin enhance hypotensive effect of diltiazem in rats. Yakugaku Zasshi. 2001;121(10):761–764.
- Ersoy R, Celik A, Yilmaz O, The effects of irbesartan and spironolactone in prevention of peritoneal fibrosis in rats. Perit Dial Int. 2007;27(4):424–431.
- Mateijsen MA, van der Wal AC, Hendriks PM, Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int. 1999;19:517–525.
- Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: Gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int. 1997;52:524–529.
- Lowry O, Rosenbraugh N, Farr L, Rondall R. (1951) Protein measurement with the folinphenol reagent. J Biol Chem. 1951;193(1):265–275.
- Tietze, F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–522.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358.
- Navarro-Gonzalves JA, Garcia-Benayas C, Arenas J. Semiautomated measurement of nitrate in biological fluids. Clin Chem. 1998;44:679–681.
- Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. New York: Academic Press; 1974:673–677.
- Racker E. Glutathione reductase (liver and yeast). In: Colowick SP, Kaplan NO, eds. Methods in Enzymology. Vol. 2. New York: Academic Press; 1955:722–729.
- Davies SJ, Bryan J, Phillips L, Russell GI. Longitudinal changes in peritoneal kinetics: The effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant. 1996;11(3):498–506.
- Kaneko K, Hamada C, Tomino Y. Peritoneal fibrosis intervention. Perit Dial Int. 2007;27(Suppl. 2):S82–S86.
- Goffin E. Peritoneal membrane structural and functional changes during peritoneal dialysis. Semin Dial. 2008;21(3): 258–265.
- Devuyst O, Topley N, Williams J. Morphological functional changes in the dialysed peritoneal cavity: Impact of more biocompatible solutions. Nephrol Dial Transplant. 2002;17(Suppl. 3):12–15.
- Saxena R. Pathogenesis and treatment of peritoneal membrane failure. Pediatr Nephrol. 2008;23(5):695–703.
- Williams JD, Craig KJ, Topley N, Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13(2):470–479.
- Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int. 2000;20(Suppl. 4):22–42.
- Gotloib L, Wajsbrot V, Shostak A. A short review of experimental peritoneal sclerosis: From mice to men. Int J Artif Organs. 2005;28(2):97–104.
- Margetts PJ, Kolb M, Yu L, Hoff CM, Gauldie J. A chronic inflammatory infusion model of peritoneal dialysis in rats. Perit Dial Int. 2001;21(Suppl. 3):S368–S372.
- Zweers MM, de Waart DR, Smit W, Struijk DG, Krediet RT. Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab Clin Med. 1999;134(2):124–132.
- Coles GA, Williams JD, Topley N. Peritoneal inflammation and long-term changes in peritoneal structure and function. In: Gokal R, Khanna R, Krediet RT, Nolph KD, eds. Textbook of Peritoneal Dialysis. 2nd ed., Oxford: Kluwer Academic Publishers; 2000:566–583.
- Di Paolo N, Gaggiotti E. Theoretical morphological approach to simple peritoneal sclerosis. Int J Artif Organs. 2005;28(2): 85–89.
- Duman S, Ozbek SS, Gunay ES, What does peritoneal thickness in peritoneal dialysis patients tell us? Adv Perit Dial. 2007;23:28–33.
- Honda K, Nitta K, Horita S, Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant. 1999;14(6):1541–1549.
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342: 1350–1359.
- Margetts PJ, Kolb M, Yu L, Inflammatory cytokines, angiogenesis, and fibrosis in the rat peritoneum. Am J Pathol. 2002;160(6):2285–2294.
- Margetts PJ, Bonniaud P. Basic mechanisms and clinical implications of peritoneal fibrosis. Perit Dial Int. 2003;23(6): 530–541.
- Garosi G, Di Paolo N. Morphological aspects of peritoneal sclerosis. J Nephrol. 2001;14(Suppl. 4):S30–S38.
- Imai H, Nakamoto H, Fukushima R, Ishida Y, Yamanouchi Y, Suzuki H. Role of adhesion molecules in the progression of peritoneal sclerosis. Adv Perit Dial. 2003;19:180–185.
- Massy ZA, Guijarro C. Statins: Effects beyond cholesterol lowering. Nephrol Dial Transplant. 2001;16(9):1738–1741.
- Oh KH, Margetts PJ. Cytokines and growth factors involved in peritoneal fibrosis of peritoneal dialysis patients. Int J Artif Organs. 2005;28(2):129–134.
- Fang CC, Yen CJ, Chen YM, Pentoxifylline inhibits human peritoneal mesothelial cell growth and collagen synthesis: Effects on TGF-b. Kidney Int. 2000;57(6):2626–2633.
- Duman S, Sen S, Sozmen EY, Oreopoulos DG. Atorvastatin improves peritoneal sclerosis induced by hypertonic PD solution in rats. Int J Artif Organs. 2005;28(2):170–176.
- Zhang W, Liu M, Wu Y, Protective effects of atorvastatin on chronic allograft nephropathy in rats. J Surg Res. 2007;143(2):428–436.
- Asberg A, Hartmann A, Fjeldså E, Holdaas H. Atorvastatin improves endothelial function in renal-transplant recipients. Nephrol Dial Transplant. 2001;16(9):1920–1924.
- Kumar S, Thuraisingham RC, Raftery MJ, Fan SL, Yaqoob MM. Statins have anti-inflammatory effects in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. 2003;14:737.
- Li C, Yang CW, Park JH, Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol. 2004;286(1):F46–F57.
- Li C, Sun BK, Lim SW, Combined effects of losartan and pravastatin on interstitial inflammation and fibrosis in chronic cyclosporine-induced nephropathy. Transplantation. 2005;79(11):1522–1529.
- Sabbatini M, Pisani A, Uccello F, Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol. 2004;15(4):901–909.
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–139 ( Review).
- Paraskevas KI. Statin therapy in peritoneal dialysis patients: Effects beyond lipid lowering. Int Urol Nephrol. 2008;40(1): 165–170.
- Goppelt-Struebe M, Hahn A, Iwanciw D, Rehm M, Banas B. Regulation of connective tissue growth factor (ccn2; ctgf) gene expression in human mesangial cells: Modulation by HMG CoA reductase inhibitors (statins). Mol Pathol. 2001;54(3): 176–179.
- Guijarro C, Egido J. Transcription factor B (NF B) and renal disease. Kidney Int. 2001;59:415–424.
- Massy ZA, Kim Y, Guijarro C, Kasiske BL, Keane WF, O'Donnell MP. Low-density lipoprotein-induced expression of interleukin-6 a marker of human mesangial cell inflammation: Effects of oxidation and modulation by lovastatin. Biochem Biophys Res Commun. 2000;267:536–540.
- Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2(1):46–53.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20(5):440–451.
- Antoniadi G, Eleftheriadis T, Liakopoulos V, Effect of one-year oral alpha-tocopherol administration on the antioxidant defense system in hemodialysis patients. Ther Apher Dial. 2008;12(3):237–242.