Abstract
There is limited information on the incidence of acute kidney injury (AKI) in patients with traumatic brain injury (TBI) although AKI may contribute to morbidity and mortality. We investigated the incidence of AKI in patients with moderate and severe TBI and the association of AKI with risk factors and outcomes in these patients.
We studied all TBI patients over 16 years of age admitted to the two designated trauma hospitals in the state of Victoria, Australia from 1 January to 31 December 2008. Patients were included if they had head trauma and presented with a Glasgow coma scale (GCS) <13. Prospectively collected data from the hospital trauma registries, ICUs, and pathology databases were analyzed retrospectively. Risk injury failure loss end (RIFLE) criteria were used to categorize renal function.
The incidence of AKI was 9.2% (19/207). Patients who developed AKI were older, had higher severity of illness scores, and a lower GCS. Overall 42.1% of these patients died in hospital compared with 18.1% in patients without AKI. In univariable linear regression analysis, age, severity of illness, and admitting hospital were associated with AKI. After multivariable logistic regression, the occurrence of AKI was associated with age (p < 0.001) and higher APACHE III scores (p = 0.016).
AKI is relatively common even in patients with TBI. Its association with age and APACHE III scores helps identify patients at higher risk of AKI.
INTRODUCTION
There is limited information on the incidence of acute kidney injury (AKI) in patients with traumatic brain injury (TBI). A few studies have broadly investigated non-neurological outcomes in TBI and reported a low incidence of ‘renal failure’ in TBI patients.Citation1–3 However, these studies used Sequential Organ Failure Assessment (SOFA) or Multiple Organ Dysfunction (MOD) scores to identify the presence of AKI and classify its severity. These scores have not been validated for AKI and use single serum creatinine (SCr) cutoffs to classify renal function, which likely only identify patients with severe AKI. Accordingly, they are insensitive to less severe changes in kidney function, which, in many studies, have been shown to be independent predictors of AKI.Citation4–6 Moreover, none of these studies focused on AKI over time, or used change in renal function from baseline to measure renal injury. This approach is now considered more appropriate than single SCr cutoff measurements.Citation7,Citation8
Recently, AKI has been defined and classified according to a consensus system called Risk injury failure loss end (RIFLE).Citation9 This system has been validated in more than 250,000 patients in multiple studies from different countries and centers, and in different patient populations.Citation7,Citation10,Citation11 The RIFLE system uses estimates of glomerular filtration rate (GFR) to assess baseline function if this is unknown and calculates changes in GFR to assess whether a patient has developed AKI. This approach has been confirmed accurate in studies involving thousands of patientsCitation7,Citation10,Citation12 and is particularly useful in populations, such as TBI patients, where a baseline SCr is typically missing.
A recent analysis of data from the Australian and New Zealand Intensive Care Society (ANZICS) adult patient database using the RIFLE criteria suggested that 11% of patients with TBI are likely to develop AKI within the first 24 hours of ICU admission.Citation10 These data, however, did not extend beyond the first day after admission. Given that the burden of AKI in patients with TBI may be greater than previously expected,Citation10 the paucity of data on the incidence of AKI in TBI,Citation1–3 the limitations of the ANZICS database study (e.g., renal function data for 24 hours only),Citation10 and the potential health resource implications, further investigation appears desirable.
In this study, the primary aim was to provide a more reliable estimate of the incidence of AKI as classified by the RIFLE criteria in patients with TBI in the state of Victoria, Australia. In addition we described their clinical characteristics stratified by AKI and investigated risk factors for AKI and associations between AKI and outcomes.
METHODS
The Alfred and Royal Melbourne Hospital Ethics Committees approved the study and waived the need for informed consent. We studied all patients, 16 years and over, with moderate or severe TBI (head injury resulting from trauma; GCS < 13), and admitted to the intensive care units of the two designated trauma centers of the state of Victoria, Australia, from 1 January 2008 to 31 December 2008. Those patients with no SCr data beyond the day of admission or the following day were excluded. Routine, prospectively collected data from the two hospital trauma registries and their intensive care unit and pathology databases were collected retrospectively.
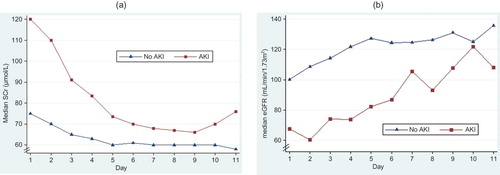
We applied the RIFLE classification system to the assessment of renal function, but did not include urine output. Because no baseline creatinine was available in these patients, their baseline GFR was estimated using normative age and gender-stratified eGFR values.Citation13 Those with no SCr measurements beyond the first 2 days were excluded to eliminate early deaths and to differentiate those who may be responsive to interventions. As the first available SCr was potentially confounded by creatinine release from muscle injury (), we used the peak SCr from the day after admission to the first 10 days in the modification of diet in renal disease (MDRD)-175 equationCitation14 to calculate the worst eGFR. Then maximal decline from baseline was calculated to establish RIFLE level of dysfunction.
STATISTICAL ANALYSIS
Associations between RIFLE categories and potential confounders were investigated using the chi-square test for categorical confounders, one way analysis of variance for normally distributed continuous confounders, and the Kruskall–Wallis test for non-normally distributed continuous confounders. Univariable relationships with the percent decline in eGFR were determined using linear regression. Multivariable models were constructed using multivariable regression analysis with both stepwise and backward elimination techniques. Comparison of the sample with and without those with no SCr measurements beyond the first 2 days was performed for descriptive, univariable, and multivariable analyses. Intercooled Stata, version 9 and above (Statacorp, College Station, Texas, USA) was used for analyses.
RESULTS
In the year 2008, we identified 216 patients fitting the inclusion criteria. Nine patients with no SCr data beyond the day of admission (making calculation of their peak level impossible) were excluded leaving a total of 207 patients. A further 20 patients with no SCr beyond the day after admission or the following day were excluded for part of the analysis. These 20 patients are referred to as ‘those with no SCr beyond day 2’ in and . Exclusion of these patients left 187 moderate or severe TBI patients with SCr values beyond the day after admission or the following day. TBI patients were relatively young and mostly male (median age 33; 76.8% males) (). Of the 20 patients with no SCr beyond day 2, 80% died, 80% were male, only 25% underwent operations, and the hospital length of stay for all but 2 of them was ≤2 days. This was reflected in the comparisons with and without exclusions ( and ).
Table 1. Patient characteristics grouped by ‘percent decline in eGFR’, including and excluding those with no SCr beyond day 2
Table 2. Comparison of univariable and multivariable estimates for linear regression models with ‘percent decline in eGFR’: including and excluding those with no SCr beyond day 2
The incidence of AKI (RIFLE Risk/Injury/Failure) was 9.2% (19/207, ). Patients with AKI tended to be older and have higher severity of illness scores and a lower Glasgow coma scale (GCS). AKI patients had a higher mortality ().
In univariable linear regression analysis, age, severity of illness, and admitting hospital were associated with decrease in eGFR; in contrast, GCS and low blood pressure were not associated with lower eGFR (). Logistic regression analysis revealed no association of AKI with mortality. In the final multivariable linear regression analysis, the percent decline in eGFR was associated with older age, higher severity of illness, and hospital of admission ().
DISCUSSION
Statement of principal findings
We conducted a retrospective epidemiological study to establish the incidence and risk factors for and outcome of AKI in patients admitted to ICU because of TBI. We found that the incidence of AKI approximates 10%. In addition we found that age, severity of illness, low GCS, and hospital of admission were risk factors for the development of AKI, and that patients who have both TBI and AKI have a mortality rate of greater than 40%.
Comparison to literature
The incidence of AKI in TBI patients was explored by Zygun et al.Citation2 who found that only 1 of 209 patients with severe TBI developed ‘renal failure’ and 7% developed ‘renal dysfunction’ (MOD score). However, these investigators used an unvalidated tool to assess for AKI, which has limited sensitivity. More recently Wahlstrom et al.Citation1 found that in a cohort of 93 TBI patients none developed severe renal failure. However, these investigators used the SOFA score criteria for AKI (creatinine ≥300 μmol/L, urine output ≤500 mL/day), which, unlike the consensus RIFLE criteria, are highly insensitive and only detect patients with the most advanced form of AKI. Bagshaw et al.Citation10 in their study on AKI in 9449 trauma patients calculated baseline SCr from the MDRD equation assuming a lower limit of normal baseline GFR (75 mL/min) which has been used in numerous studiesCitation7,Citation12,Citation15–17 and found that, in the first 24 hours, the incidence of AKI was 11%, a finding similar to that reported in this study. The RIFLE criteria measure change in renal function from a baseline value,Citation9 have been extensively used and validated to classify renal function in studies cumulatively involving >250,000 subjects,Citation6,Citation7,Citation10 and seem appropriate for this kind of assessment. Our study using a similar methodology found similarly that the incidence of AKI in TBI patients is approximately 10%. No other studies have defined the risk factors for AKI in this population. Indeed, recent experimental evidenceCitation18 confirming prevention of renal injury as a result of the neuroprotective effect of two interventions (erythropoietin and carbamylated erythropoietin) given in the presence of brain death makes quantification of the extent of AKI in patients with brain injury even more relevant.
Implications of study findings
AKI can worsen cerebral edema,Citation19 and is associated with a greater risk for death.Citation6 Severe cases require costly treatments and can result in long-term damaging effects on the kidneys.Citation20 Accordingly, it is desirable to prevent development of AKI. The observation that older patients are at a greater risk of its development () suggests the need to be particularly focused on the resuscitation efforts of older patients with TBI. The high mortality seen in these patients allows appreciation of the fact that the presence of AKI in these patients is also an additional predictor of poor outcome.
The association of AKI with a lower GCS () may reflect the severity of trauma and its impact on the kidneys. However, AKI can also directly contribute to worsen cerebral edema. The fact that the injury severity score (an indication of the severity of trauma) is not associated with AKI ( and ) suggests that the association of AKI with GCS may be direct and/or due to its predictive ability of poor outcome.
Notably, patients without AKI tended to have a significant improvement in eGFR of approximately 20% (). This effect may likely follow fluid resuscitation and possible related changes in GFR induced by it. However, it may also represent a dilutional effect on SCr induced by fluid administration (pseudo-increase in GFR) or both dilution and related GFR changes. These dynamic effects highlight the difficulties associated with evaluating renal function in trauma patients.
Strengths and limitations
The strength of this study is that it uses a sufficiently large cohort with prospectively collected data and electronically collected and stored laboratory data and applies such data to the estimation of the incidence of AKI using modern and validated criteria (RIFLE). As the sample is young (median age 33) which characterizes TBI patientsCitation21 and given that TBI patients tend to have reasonable pre-morbid health, in the absence of baseline information, we sought a normative baseline eGFR (males 85, females 83) in accordance with a healthy young population.Citation13 Furthermore, we used percent decline in eGFR from ‘normative’ baseline to worst eGFR in the first 10 days, in preference to estimating baseline SCr given recent doubts surrounding this method.Citation22 This methodology has to be taken into account when interpreting the findings. However, despite concerns that the use of eGFR is not accurate when patients are not in steady state, it has been used in this context and still predicts association with known risk factors and outcomes.Citation10,Citation23–25
The cohort is from two hospitals in the state of Victoria, Australia, and may not apply to other jurisdictions. However, the Victorian trauma system has many similarities with other trauma systems elsewhere in the developed world.
Between hospitals there was a 16 μmol/L difference in mean/median SCr. While there were some differences in characteristics of hospital samples, this is probably largely because of differences in SCr measurement methodology. This likely laboratory effect was adjusted for in multivariable linear regression models.
The pattern of SCr levels was typically elevated in the first available SCr level followed by a dip (). The early peak may have been due to muscular injury caused by trauma or decreased perfusion pressure causing an increase in muscle breakdown and higher SCr.
Although the data were collected prospectively as part of routine data collection during 2008, this is a retrospective analysis and therefore control over quality of the data is limited. Where duplicate data were available from two or more sources, checks revealed high reliability of the data.
Future research
The presence of AKI in 10% of patients and its association with increased mortality allow the identification of whether pharmacologic interventions which appear to be beneficial in neurotrauma can simultaneously protect brain and kidney and decrease the incidence of AKI. Erythropoietin and carbamylated erythropoietin are two such treatments which have been shown to protect the brain and kidney in brain dead rats because of inhibition of the inflammatory processes caused by brain injury.Citation18 Their potential for a similar dual effect in patients with TBI is worthy of investigation. Such studies may provide useful insights into AKI in this setting and be of relevance to other settings.
CONCLUSIONS
AKI is relatively common even in patients with TBI and identifies patients with a high risk of death. Its association with age, hypotension, and APACHE III scores helps identify patients at higher risk of AKI which may be targets of future trials of drugs that are simultaneously neuroprotective and nephroprotective.
Acknowledgments
We thank the Royal Melbourne and Alfred Hospital Trauma Registries, ICU departments, and Pathology departments for their collaboration in providing data for this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wahlstrom MR, Olivecrona M, Nystrom F, Koskinen LO, Naredi S. Fluid therapy and the use of albumin in the treatment of severe traumatic brain injury. Acta Anaesthesiol Scand. 2009;53:18–25.
- Zygun D, Kortbeek J, Fick G, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33:654–660.
- Zygun DA, Doig CJ, Gupta AK, Non-neurological organ dysfunction in neurocritical care. J Crit Care. 2003;18:238–244.
- Cooperative Antimicrobial Therapy of Septic Shock Database Research Group, Bagshaw SM, Lapinsky S, et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881.
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974.
- Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–546.
- Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23: 1203–1210.
- Ricci Z, Ronco C. Today's approach to the critically ill patient with acute kidney injury. Blood Purif. 2009;27:127–134.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8:R204–R212.
- Bagshaw SM, George C, Gibney RT, Bellomo R. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail. 2008;30:581–589.
- Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettilä V. Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–546.
- Ostermann M, Chang RWS. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35: 1837–1843.
- Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int. 2007;72:632–637.
- Mathew TH, Johnson DW, Jones GRD. On behalf of the Australasian Creatinine Consensus Working Group. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: Revised recommendations. Med J Aust. 2007;187:459–463.
- Guitard J, Cointault O, Kamar N, Acute renal failure following liver transplantation with induction therapy. Clin Nephrol. 2006;65:103–112.
- Heringlake M, Knappe M, Vargas Hein O, Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006;72:645–654.
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917.
- Nijboer WN, Ottens PJ, van Dijk A, van Goor H, Ploeg RJ, Leuvenink HG. Donor pretreatment with carbamylated erythropoietin in a brain death model reduces inflammation more effectively than erythropoietin while preserving renal function. Crit Care Med. 2010;38:1155–1161.
- Davenport A. Renal replacement therapy in the patient with acute brain injury. Am J Kidney Dis. 2001;37:457–466.
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16: 3365–3370.
- Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A. The effect of gender on patients with moderate to severe head injuries. J Trauma. 2009;67:950–953.
- Bagshaw SM, Uchino S, Cruz D, A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24:2739–2744.
- Cooper WA, O'Brien SM, Thourani VH, Impact of renal dysfunction on outcomes of coronary artery bypass surgery – results from the society of thoracic surgeons national adult cardiac database. Circulation. 2006;113:1063–1070.
- Hillis GS, Croal BL, Buchan KG, Renal function and outcome from coronary artery bypass grafting – impact on mortality after a 2.3-year follow-up. Circulation. 2006;113: 1056–1062.
- Bagshaw SM, Uchino S, Bellomo R, Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24:129–140.