Abstract
Nighttime systolic blood pressure (BP) from ambulatory blood pressure monitoring (ABPM) is more predictive than clinic BP for cardiovascular disease, stroke, and death even after controlling for clinic BP. However, ABPM is expensive and burdensome to obtain regularly. BPs obtained in the hospital may provide a window into nighttime BP. We conducted a retrospective cohort study of all hypertensive patients admitted to the Louis Stokes Cleveland Department of Veterans Affairs Medical Center (LSCDVAMC) in 2002 and 2003 with one or more BP recorded between midnight and 6 am on the day of or the day before discharge. The mean age of the study population (n = 1085) was 62 years and 96% were male. Twenty-two percent had coronary artery disease (CAD) and 34% had diabetes. The mean nighttime systolic BP was 132 mmHg and baseline glomerular filtration rate (GFR) was 83 mL/min per 1.73 m2. Over a median follow-up period of 4.3 years, 266 subjects died, 22 developed end-stage renal disease (ESRD), 99 had a 50% decline in GFR, and 136 developed myocardial infarction (MI). The adjusted hazard ratios (HRs) associated with a 10 mmHg increase in nighttime systolic BP were 1.03 (95% confidence interval, 0.93–1.15) for death, 1.30 (0.94–1.80) for ESRD, 1.26 (1.08–1.47) for a 50% decline in GFR, 1.07 (0.92–1.23) for myocardial infarction, and 1.12 (1.03–1.23) for a composite of death, ESRD, or a 50% decline in GFR. In conclusion, nighttime systolic BP in hospitalized patients is an independent predictor of important clinical outcomes such as a composite of death, ESRD, or a 50% decline in GFR.
INTRODUCTION
Although clinic-based blood pressure (BP) has been the standard of care for clinical practice and research, there has been increasing interest in out of office readings such as ambulatory BP monitoring (ABPM) and home BP monitoring. This is driven by the fact that measurement of clinic BP has a number of limitations. First, clinic BP is subject to the “white-coat” effect – it is elevated in up to 20–35% of patients who are otherwise normotensive.Citation1 Clinic BP is unable to detect masked hypertension, a condition in which BP is normal in the office but high outside the office.Citation2 Clinic BP is also unable to measure diurnal BP variation. Finally, other methods for assessing BP, such as ABPM, may provide more accurate estimates of risk for cardiovascular disease, stroke, end-stage renal disease (ESRD), and death even after controlling for clinic BP.Citation3–16
It is well known that BP varies during the course of the day and night.Citation17 In recent studies, it is clear that nighttime BP is a powerful predictor of long-term outcomes in hypertensive patients.Citation3–6,Citation15,Citation18 Nighttime BP is typically measured by ABPM. Another opportunity to measure BP at night is in hospitalized patients; BPs are checked several times a day in hospitalized patients. BP obtained in the hospital may provide a window into nighttime BP, albeit possibly awake nighttime BP. To our knowledge, there have been no studies evaluating the ability of nighttime BP in hospitalized patients to predict the risk of adverse clinical outcomes. Hospital nighttime BPs offer an opportunity to evaluate the predictive value of nighttime BP in a setting that has not been previously studied.
The objective of this study was to assess whether nighttime BP measured in the hospital setting predicts subsequent risk of death, ESRD, decline in glomerular filtration rate (GFR), and myocardial infarction (MI).
MATERIALS AND METHODS
We performed a retrospective cohort study of all patients admitted to the Louis Stokes Cleveland Department of Veterans Affairs Medical Center (LSCDVAMC) hospital in 2002 and 2003. The study was approved by the Institutional Review Board at the LSCDVAMC. A database query tool was used to collect demographic, vital status, past medical history, BP measurements, and laboratory data from the VA clinical database. Inclusion criteria were a history of hypertension, at least one outpatient BP measurement within 18 months prior to admission, and at least one BP recorded between midnight and 6 am on the day of or the day before discharge. The date of discharge was used to minimize the effects of acute illness on BP. For each subject, the index admission was the first admission in 2002 or 2003.
Hypertension was defined by the presence of 2 or more ICD-9 codes 401–405.xx prior to or including the index admission, a BP > 140/90 mmHg on two or more occasions prior to the index admission, or a prescription for an antihypertensive medication in the 12 months prior to the index admission.Citation1
Clinic systolic BP was defined as the most recent systolic BP within 18 months prior to the index admission or the average of the most recent two if more than one BP were available. Nighttime systolic BP was defined as the average of all systolic BP between midnight and 6 am on the day of or the day before the index discharge. Hospital BP was measured by nurses and nursing assistants using automated BP machines, typically on awake subjects. Dipping was defined as the ratio of average nighttime to average daytime systolic BP obtained on the day of or the day before discharge. The simplified MDRD (Modification of Diet in Renal Disease) study equation was used to estimate GFR (mL/min per 1.73 m2) according to the following formula: 186.3 × (serum creatinine in mg/dL−1.154) × (age in years−0.203) × 1.212 (if black) × 0.742 (if female).Citation19 Baseline GFR was defined as the lower of (a) the average of the last two outpatient GFRs within 18 months prior to the index admission or (b) the last hospital GFR.
Exclusion criteria were the presence of ESRD, age greater than 80 years at the time of admission, heart failure (HF), length of stay greater than 30 days, and death or loss to follow-up within 60 days after discharge.
Patients with ESRD were identified through chart review of all subjects with either a GFR less than 15 mL/min per 1.73 m2 or an ICD-9 code of 585.5, 585.6, 585.9, 792.5, V42.0, V45.1, V45.6, V56.2, V56.31, V56.32, or V56.8 prior to the index discharge date. ESRD was defined as the presence of chronic dialysis or transplant prior to the index discharge date. HF was defined by the presence of two or more of the following ICD-9 codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 414.8 or between 428 and 428.xx. Diabetes was defined as two or more ICD-9 codes (250–250.xx) or a prescription for an oral hypoglycemic medication prior to the index admission. A history of coronary artery disease (CAD) was defined by the presence of two or more of the following ICD-9 codes: 410–414.xx or 429.2. Cancer was defined as two or more ICD-9 codes using the Agency for Healthcare Research and Quality Clinical Classification Software.Citation20 The sensitivity and specificity of VA administrative ICD-9 codes for patient comorbidities has been shown to be greater than 90–95%.Citation21,Citation22
OUTCOMES
The primary outcomes were death, ESRD, a 50% decline in GFR, and MI. These outcomes were chosen because they are clinically significant and events are identifiable in the VA clinical database.
Death was ascertained by the presence of a date of death in the VA clinical database or a provider note indicating the patient was deceased. In the latter case, the date of death was determined to be the date of the note unless a specific date was indicated. The last date of follow-up was 31 December 2007 for subjects with any laboratory value or vital sign after that date. For subjects without a date of death or a laboratory value or vital sign after 31 December 2007, a manual review of the clinical record was performed to determine the last date of follow-up. The VA clinical database has been found to be highly accurate with regard to the vital status when compared to the official state death records with a sensitivity and specificity greater than 91% and 99%, respectively.Citation23
ESRD was defined by the presence of chronic dialysis or the receipt of a kidney transplant as determined by chart review. Charts were reviewed for all subjects with either a GFR less than 15 mL/min per 1.73 m2 or an ESRD ICD-9 code (see above). A 50% decline in GFR was defined as the date of the first outpatient GFR 50% less than baseline. Censoring for the renal outcomes of ESRD and a 50% decline in GFR was defined as the date of the last available GFR.
For patients without a history of CAD, MI was defined as the presence of two or more CAD ICD-9 codes (see above) after the index admission with the date of the first ICD-9 serving as the event date. For subjects with a history of CAD, MI was determined through the chart review of all subjects with a positive troponin. MI was defined as a positive troponin plus (1) symptoms of ischemia; (2) new ST-T changes, new left bundle branch block, or development of pathological Q waves on ECG; or (3) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.Citation24 Two investigators (P.D. and N.R.) reviewed the medical records for the 141 subjects with a positive troponin to evaluate for the presence or absence of MI and the date of the event if present. In the case of disagreement (n = 4 subjects), a third investigator's (M.R.) blinded opinion was used for event ascertainment.
STATISTICS
Baseline characteristics are reported as mean (SD) and as percentages. Univariate and multivariable Cox proportional hazards models were used to determine the effect of nighttime and clinic systolic BP on the time to each outcome. For each outcome, models were constructed for nighttime and clinic systolic BP separately. Covariates included age, gender, race, length of stay, diabetes, CAD, cancer, tobacco use, increase in creatinine during index hospitalization, ratio of nighttime to daytime hospital BP (dipping), baseline GFR, albumin, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, calcium, hemoglobin, and proteinuria. To investigate whether nighttime BP was an independent risk factor, a final adjusted model for each outcome was constructed that included both nighttime and clinic systolic BP and all previous covariates. For each model, single imputation was performed to account for any missing data. Model assumptions were also assessed in a variety of ways. Specifically, (1) informative censoring was evaluated by performing Kaplan–Meier analyses of censored times; (2) the assumed linear relationship between nighttime and clinic systolic BP and the log hazard of each outcome was assessed using restricted cubic splines; (3) the proportional hazards assumption was evaluated using Schoenfeld residuals; and (4) each model was also assessed by examining deviance residuals and evaluating outliers. Results are reported as the hazard ratio (HR) for a 10 mmHg increase in nighttime or clinic systolic BP. All analyses were conducted using R version 2.6.1 (http://www.r-project.org).
RESULTS
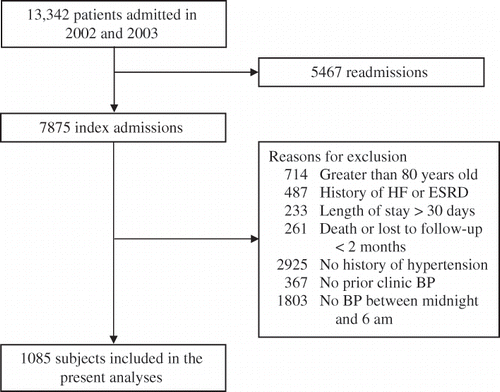
There were 7875 patients admitted to the LSCDVAMC Wade Park hospital in 2002 and 2003. One thousand eighty-five subjects met the inclusion criteria and formed the basis for further analyses. Other patients were excluded because of the presence of one or more exclusion criteria as shown in .
Baseline characteristics for the cohort are shown in . The mean (SD) age of the study population was 62 years,Citation11 96% were male, and 40% were African–American. One nighttime BP measurement was available for 571 (52%) subjects, two measurements were available for 459 (42%) subjects, and 58 (5%) subjects had three or four eligible nighttime BPs recorded. The median (25th, 75th percentile) time of nighttime BP measurement was 3:00 am (1:54 am, 4:48 am). The mean clinic systolic BP was 141.5 (17.7) mmHg, nighttime systolic BP was 132 (20) mmHg, and baseline GFR was 83 (31) mL/min per 1.73 m2. Twenty-two percent had CAD and 34% had diabetes. The median (25th, 75th percentile) interval between the index hospitalization and the preceding clinic BP was 42 (20.4, 81.7) days.
Table 1. Baseline characteristics for subjects with and without death, ESRD, or 50% decline in GFR
OUTCOMES
Over a median (25th, 75th percentile) follow-up period of 4.3 (3.3, 4.7) years, 266 (24.5%) subjects died, 22 (2.1%) developed ESRD, 99 (9.4%) had a 50% decline in GFR, and 136 (12.5%) developed MI (). The relationship between nighttime and clinic systolic BP and most of the outcomes was found to be nonlinear. Therefore, analyses were conducted using piecewise linear regression with a knot, determined by restricted cubic splines, at 130 mmHg for nighttime systolic BP and 140 mmHg for clinic systolic BP. Upon examination of the association between censoring times and key exposures, no evidence of informative censoring was found and, therefore, standard Cox proportional hazard models were fit. The proportional hazards assumption was met in all models and there were no significant deviance residuals or outliers.
Table 2. Number of events by outcome
The unadjusted and adjusted HRs associated with a 10 mmHg increase in nighttime systolic BP above 130 mmHg and clinic systolic BP above 140 mmHg and death, ESRD, a 50% decline in GFR, and MI are shown in . Neither nighttime nor clinic systolic BP predicted an increased risk of death (unadjusted HR 1.02, p = 0.73 and 1.01, p = 0.86) or MI (unadjusted HR 1.11, p = 0.12 and 1.07, p = 0.42). Nighttime and clinic systolic BP both predicted an increased risk of ESRD (unadjusted HR 1.65, p < 0.001 and 1.42, p = 0.03). However, nighttime but not clinic BP remained marginally significant after adjustment (adjusted HR 1.30, p = 0.11). Nighttime and clinic systolic BP both predicted an increased risk for a 50% decline in GFR (unadjusted HR 1.33, p < 0.001 and 1.29, p = 0.003), but only nighttime systolic BP remained significant after adjustment (adjusted HR 1.26, p = 0.003). Both nighttime and clinic systolic BP predicted the composite outcomes of death, ESRD, or a 50% decline in GFR (unadjusted HR 1.12, p = 0.007 and 1.11, p = 0.06); and death, MI, ESRD, or a 50% decline in GFR (unadjusted HR 1.10, p = 0.009 and 1.10, p = 0.04). Again, however, only nighttime systolic BP predicted the composite outcomes after adjustment (adjusted HR 1.12, p = 0.01 and 1.10, p = 0.03).
Table 3. Unadjusted and adjusted hazard ratios for death, ESRD, a 50% decline in GFR, and myocardial infarction associated with a 10 mmHg increase in nighttime SBP above 130 mmHg and clinic SBP above 140 mmHg
Given the nonsignificant relationship between nighttime BP and MI, a post hoc analysis was performed evaluating the relationship in those with and without CAD at baseline. In patients with a history of CAD, there was no significant relationship between nighttime BP and MI. However, in patients without a history of CAD, the relationship between nighttime BP and MI was marginally significant [unadjusted HR 1.13 (95% confidence interval, 0.97–1.31), p = 0.12; fully adjusted HR 1.13 (0.95–1.33), p = 0.16]. In addition, because the relationship between nighttime BP and death was nonsignificant, a second post hoc analysis was performed evaluating the relationship between dipping status (percent change in hospital systolic BP from day to night) and death. Subjects above the median dipping percentage (nondippers) were found to be at an increased risk for death [unadjusted HR 1.32 (0.99–1.75); p = 0.059], but the relationship was no longer significant after adjustment [HR 1.16 (0.86–1.56); p = 0.32].
DISCUSSION
These data are the first to show that nighttime systolic BP measured in hospitalized patients is an independent predictor of a 50% decline in GFR and a composite of death, ESRD, or a 50% decline in GFR. This novel finding adds to a growing body of evidence supporting nighttime systolic BP as a useful predictor of end-organ damage and adverse clinical outcomes in patients with hypertension.
Most studies have found ABP to be more predictive than clinic BP for cardiovascular disease, stroke, and death even after controlling for clinic BP.Citation3–15 A similar trend indicates that nighttime ambulatory BP (ABP) provides more prognostic information than daytime ABPCitation3–6,Citation15 with the exception of one cohort.Citation9 However, the relationship between nocturnal BP and renal outcomes has only been evaluated in a few cohorts. Nondipping has been shown to be a risk factor for the development of microalbuminuria, an increase in urinary protein excretion, and a decline in creatinine clearance.Citation25–27 Furthermore, in a cohort of 217 Veterans with chronic kidney disease (CKD) followed for a median of 3.5 years, nondipping was found to be a significant predictor for ESRD independent of clinic BP.Citation16 Data from the African American study of Kidney Disease and Hypertension (AASK) indicate that a large majority of seemingly well-controlled (by office BP) hypertensive patients had elevated BP at night; elevated nocturnal BP was associated with more severe manifestations of target organ damage.Citation28
Our data confirm these previous findings by demonstrating a significant relationship between nighttime systolic BP and a decline in GFR and a composite of death, ESRD, or a decline in GFR. Furthermore, the point estimate (adjusted HR 1.30) indicates a higher risk of ESRD associated with elevated nighttime systolic BP. Although the relationship between ESRD and nighttime BP was only marginally significant, this is likely a result of the limited number of events.
In addition, our study, using a novel approach to ascertain nighttime BP, corroborates previous studies indicating the significant association between nighttime BP and adverse clinical outcomes. To our knowledge, this is the first study to evaluate the long-term predictive value of BP measurements obtained in the hospital setting. This is particularly relevant to nocturnal BP, because daytime BPs are easily obtained either in clinic or at home, whereas nighttime BP typically requires ABPM, which presents logistical challenges. This study is the first demonstration of the predictive value of nighttime BP without the use of ABPM and serves as an additional “proof of principle” of the importance of nighttime BP as a marker of long-term prognosis. As there is increasing recognition of the importance of nocturnal BP, novel ways of measuring BP at night need to be developed. ABPM remains the gold standard but is cumbersome, expensive, and may be poorly reproducible. Our study indicates that hospitalized BP provides another window into patients' nighttime BP.
CKD has been shown to be a risk factor for elevated nighttime BP.Citation29–31 The mechanism by which CKD leads to elevated nighttime BP is not well understood but is likely multifactorial and includes volume-dependent hypertension exacerbated by recumbent posture, abnormal sodium handling, and comorbidities such as diabetes and autonomic insufficiency. Cardiac autonomic neuropathy, one aspect of autonomic insufficiency, is associated with elevated nighttime BP in patients with and without CKD.Citation32–35 Patients with CKD are known to have increased sympathetic activity.Citation36,Citation37 One possible mechanism for increased nighttime BP in subjects with cardiac autonomic neuropathy is an increased sympathetic activation causing increased renin release and proximal tubular sodium reabsorption.Citation38–40
Unlike previous studies, we did not find a significant relationship between nighttime BP and either MI or death. This discrepancy could be due to the retrospective nature of our study and an inability to accurately capture all events, leading to misclassification. However, vital status in the VA clinical database is 91% sensitive.Citation23 Additionally, sensitivity in our study was increased through a manual chart review for all subjects without a date of death, laboratory value, or vital sign after the predetermined last date of follow-up. Post hoc analyses did indicate a trend toward a significant association between dipping status and death and between nighttime BP and MI in patients without a history of CAD.
Our study has a number of strengths. We are the first to report the affect of nighttime BP obtained in hospitalized patients on the risk for adverse clinical events. We studied a large cohort of patients and carefully adjudicated a number of important objective clinical end-points. Finally, we controlled for most of the known cardiovascular and renal risk factors including age, diabetes, CAD, tobacco use, proteinuria, baseline GFR, albumin, cholesterol, hemoglobin, and clinic BP. We also controlled for nondipping, a risk factor known to be associated with cardiovascular events, decline in GFR, left ventricular hypertrophy, stroke, and death.Citation3,Citation5,Citation6,Citation11,Citation15,Citation41–44
A number of limitations should be considered when evaluating our results. First, the cohort is nearly all male, reflecting the current VA inpatient population. Second, the study was retrospective. Nevertheless, we were able to collect information on nearly all of the established risk factors for cardiovascular and renal disease. Third, BPs obtained in the hospital were not standardized or collected using rigorous research methods and are prone to misclassification. However, any misclassification was likely nondifferential. Additionally, we did not include HF or stroke as outcomes, both of which have been shown to be associated with nighttime ABP. In addition, there is an increased likelihood that the hospitalized patients in this cohort were awake at the time of their BP measurements which may affect the relationship between nighttime BP and adverse clinical outcomes.Citation45 Furthermore, data on antihypertensive treatment during the hospitalization, which may have affected nighttime BP, were unavailable. Finally, a number of factors may influence hospital nighttime BP such as acute illness, sleep disturbances, and white-coat hypertension; it is unclear how these hospital BPs correlate with ambient nighttime sleep BPs.
CONCLUSIONS
With this study, we have demonstrated that nighttime systolic BP in hospitalized patients is an independent risk factor for a 50% decline in GFR and a composite of death, ESRD, or a 50% decline in GFR. These results are further validation of the importance of nighttime BP as a risk factor for adverse clinical outcomes, even when BP is measured using a novel approach. A better understanding of the pathophysiology of elevated nighttime BP may lead to specific therapies to lower nighttime ambulatory BP and reduce the associated morbidity and mortality.
Acknowledgments
This work was presented at the American Society of Nephrology meeting in Philadelphia, Pennsylvania, USA on 8 November 2008.
Declaration of interest: The study was funded partly through NIH training grant 5T32DK007470-23 (P.E.D.). Dr. Rahman reports receiving grant support from NIH and King pharmaceutical and honoraria from Boehringer-Ingelheim. Drs. Drawz, Rosenthal, and Babineau report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Chobanian AV, Bakris GL, Black HR, Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252.
- Padiyar A, Rahman M. Ambulatory blood pressure monitoring: An argument for wider clinical use. Cleve Clin J Med. 2007;74(11):831–838.
- Fagard RH, Celis H, Thijs L, Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1): 55–61.
- Dolan E, Stanton A, Thijs L, Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension. 2005;46(1): 156–161.
- Staessen JA, Thijs L, Fagard R, Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic hypertension in europe trial investigators. JAMA. 1999;282(6): 539–546.
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: Unique aspects of blood pressure during sleep. Hypertension. 2007;49(6):1235–1241.
- Clement DL, De Buyzere ML, De Bacquer DA, Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003; 348(24):2407–2415.
- Khattar RS, Swales JD, Banfield A, Dore C, Senior R, Lahiri A. Prediction of coronary and cerebrovascular morbidity and mortality by direct continuous ambulatory blood pressure monitoring in essential hypertension. Circulation. 1999; 100(10):1071–1076.
- Ohkubo T, Hozawa A, Nagai K, Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: The Ohasama study. J Hypertens. 2000;18(7):847–854.
- Ohkubo T, Imai Y, Tsuji I, Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: A pilot study in Ohasama. J Hypertens. 1997;15(4):357–364.
- Verdecchia P, Porcellati C, Schillaci G, Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793–801.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: A prospective study. Hypertension. 1998;31(2):712–718.
- Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and mortality: A population-based study. Hypertension. 2005;45(4):499–504.
- Bjorklund K, Lind L, Zethelius B, Andren B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107(9):1297–1302.
- Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295(24):2859–2866.
- Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(7):1175–1180.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368–2374.
- Boggia J, Li Y, Thijs L, Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet. 2007;370(9594):1219–1229.
- Levey AS, Greene T, Kusek J, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828.
- AHRQ Clinical Classification Software. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed August 05, 2010.
- Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: What's the optimal approach? Am J Med Qual. 2004;19(5):201–206.
- Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care. 2002;8(1):37–43.
- Dominitz JA, Maynard C, Boyko EJ. Assessment of vital status in Department of Veterans Affairs national databases. Comparison with state death certificates. Ann Epidemiol. 2001;11(5):286–291.
- Thygesen K, Alpert JS, White HD, Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653.
- Lurbe E, Redon J, Kesani A, Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347(11):797–805.
- Timio M, Venanzi S, Lolli S, “Non-dipper” hypertensive patients and progressive renal insufficiency: A 3-year longitudinal study. Clin Nephrol. 1995;43(6):382–387.
- Palmas W, Pickering T, Teresi J, Nocturnal blood pressure elevation predicts progression of albuminuria in elderly people with type 2 diabetes. J Clin Hypertens (Greenwich). 2008;10(1):12–20.
- Pogue V, Rahman M, Lipkowitz M, Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20–27.
- Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12(11):2301–2307.
- Fukuda M, Munemura M, Usami T, Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004; 65(2):621–625.
- Paoletti E, Bellino D, Amidone M, Rolla D, Cannella G. Relationship between arterial hypertension and renal damage in chronic kidney disease: insights from ABPM. J Nephrol. 2006;19(6):778–782.
- Ragot S, Herpin D, Siche JP, Ingrand P, Mallion JM. Autonomic nervous system activity in dipper and non-dipper essential hypertensive patients. What about sex differences? J Hypertens. 1999;17(12 Pt 2):1805–1811.
- Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26(5):808–814.
- Liu M, Takahashi H, Morita Y, Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in hemodialysis patients. Nephrol Dial Transplant. 2003;18(3):563–569.
- Hojo Y, Noma S, Ohki T, Nakajima H, Satoh Y. Autonomic nervous system activity in essential hypertension: A comparison between dippers and non-dippers. J Hum Hypertens. 1997; 11(10):665–671.
- Ligtenberg G, Blankestijn PJ, Oey PL, Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999;340(17):1321–1328.
- Schlaich MP, Socratous F, Hennebry S, Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009; 20(5):933–939.
- Stella A, Zanchetti A. Effects of renal denervation on renin release in response to tilting and furosemide. Am J Physiol. 1977;232(5):H500–H507.
- Thames MD, DiBona GF. Renal nerves modulate the secretion of renin mediated by nonneural mechanisms. Circ Res. 1979;44(5):645–652.
- Bell-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest. 1976;57(4):1104–1107.
- Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006; 166(8):846–852.
- Ohkubo T, Hozawa A, Yamaguchi J, Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The Ohasama study. J Hypertens. 2002;20(11):2183–2189.
- Verdecchia P, Schillaci G, Guerrieri M, Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81(2):528–536.
- Verdecchia P, Schillaci G, Gatteschi C, Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation. 1993;88(3): 986–992.
- Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007; 49(4):777–783.