Abstract
Background and Objective: Glutathione S-transferases (GSTs) belong to a family of ubiquitous and multifunctional enzymes that work as one of the endogenous antioxidants in our body. This study was designed to look into the association of GST polymorphism with oxidative stress in both diabetic and nondiabetic chronic kidney disease (CKD). Design and Methods: Three groups of patients (50 in each): diabetics without CKD (DM), diabetic CKD (DM-CKD), and nondiabetic CKD (NDM-CKD) and 50 age- and sex-matched healthy controls were recruited. Genotyping was done for GSTM1 and GSTT1 genes using a multiplex polymerase chain reaction. Serum GST and malondialdehyde (MDA) as a marker of oxidative stress were measured spectrophotometrically. Results: Based on genotyping, subjects were categorized as GSTM1+/GSTT1+, GSTM1−/GSTT1+, GSTM1+/GSTT1−, and GSTM1−/GSTT1−. Serum GST levels were lower among subjects with deletion in one/both GST genes, whereas MDA levels were found to be correspondingly raised. A negative correlation for MDA versus GST levels was observed among genotypes with one/both gene deletions. Presence of GSTM1+/GSTT1− and GSTM1−/GSTT1− was significantly higher among patients with CKD in both diabetics and nondiabetics. Interpretations and Conclusions: GSTM1 and GSTT1 deletions singly or together were associated with lower GST levels and higher oxidative stress in both diabetic and nondiabetic CKD. Interestingly, GSTT1 deletion appears to be associated with both diabetic and nondiabetic CKD irrespective of the GSTM1 status.
INTRODUCTION
Chronic kidney disease (CKD) has become a major cause of morbidity and mortality and is considered a significant public health problem that places a significant burden on global health care resources. Despite relentless research, the complex etiopathogenesis of CKD has not been fully understood. Because of the heterogeneity involved in the etiology of CKD, which ranges from infectious and multisystem metabolic diseases on one hand to congenital and genetic disorders on the other, it has never been easy to identify the underlying mechanisms involved in the pathogenesis. Nevertheless, few studies have shown that oxidative stress might play an important role in the pathogenesis of CKD because it is increased in CKD patients.Citation1,Citation2 Noteworthy, in this regard is the fact that oxidative stress is also considered to be the final common pathway for the development of diabetic complications including nephropathy and hence can become an important confounding variable because diabetes mellitus (DM) has been implicated as the single largest cause of CKD.Citation3,Citation4 Several factors are responsible for the regulation of the balance between pro-oxidants and antioxidants in the body. The glutathione S-transferases (GSTs) (EC 2.5.1.18) belong to a family of ubiquitous and multifunctional enzymes that work as one of the endogenous antioxidants through their ability to catalyze the conjugation of reduced glutathione with electrophilic compounds and through their glutathione peroxidase activity.Citation5,Citation6 Hence, reduced GST expression may result in diminished capacity of defense against oxidative stress. Interestingly, an earlier study has documented over-expression of GSTs in erythrocytes of CKD patients pointing to the fact that this group of enzymes might be involved in the pathogenesis of CKD.Citation7
Human GSTs are divided into various classes and subclasses. Among the different subclasses, in human kidney GST μ class is mainly localized in the tubules whereas GST θ class is highly expressed in liver and kidney.Citation8 Both these genetic loci are known to be highly polymorphicCitation9 and some of these polymorphisms lead to change in the expression of the enzyme, either qualitatively or quantitatively and hence can render individuals susceptible to various diseases including CKD.Citation10,Citation11 Among the various known genetic polymorphisms of GST, the GSTM1 and GSTT1 genes are the most widely studied, mainly because the null variants of these genes might result in reduced GST expression leading to reduced antioxidant defense. Furthermore, they have been shown to be present in healthy Indian population in significant frequencies in some recent reports,Citation12,Citation13 including one from our laboratory. In the past few years, several studies have shown the association of genetic polymorphisms of GST with the development of CKD, mostly of diabetic origin, but without any conclusive results.Citation9,Citation14,Citation15 Although Fujita et al.Citation14 found no association of GSTM1 deletion with diabetic nephropathy in Japanese type 2 DM patients, Yang et al. showed that GSTT1 null genotype was a risk factor for the development of diabetic nephropathy in the Chinese population. In 2005, Kim et al.Citation15 reported that GSTM1 null genotype is associated with type 2 diabetic nephropathy in the Korean population. We have come across only one previous report studying the GST polymorphisms in relation to CKD where the authors concluded that the null polymorphism of the detoxifying enzymes, GSTT1 and GSTM1, were associated with the risk of developing end-stage renal disease (ESRD).Citation16
The mechanism by which GST polymorphism leads to CKD is not well-understood. In a previous study, Hayek et al.Citation17 have shown that GSTT1 null genotype is associated with increase in markers of lipid peroxidation among diabetics. A recent study from our laboratory has also demonstrated the role of GST polymorphism in the development of oxidative stress in preterm labor cases.Citation18 This study was designed to investigate the role of the GST polymorphism in determining variation in susceptibility of individuals to CKD and to pinpoint the probable underlying mechanism.
METHODS
Study design
This study was a cross-sectional, case-control study designed to investigate the association of GST polymorphism with oxidative stress in CKD patients. Written informed consent was obtained from all the recruits and ethical approval was obtained from Institutional Ethical Committee for Human Research as per guidelines.
Patients
The study groups comprised 50 healthy subjects as controls and three groups of 50 patients each, based on presence or absence of DM and CKD. The first group comprised patients with history of type 2 DM for at least 10 years without any microalbuminuria or overt proteinuria. The second group comprised individuals with type 2 DM with nephropathy (DM-CKD), defined by the presence of microalbuminuria or overt proteinuria in the absence of urinary tract infection, having DM for more than 5 years and evidence of diabetic retinopathy. The third group of patients included individuals with evidence of overt proteinuria and/or deranged renal function for more than 3 months in the absence of DM and any systemic or local infection (NDM-CKD). Peripheral venous blood samples were collected from the recruits and used for genotypic and biochemical studies. For patients on serial hemodialysis, pre-dialytic blood samples were collected. All subjects were age- and sex-matched nonsmokers. Care was taken not to include known relatives of patients as controls in this study.
Biochemical analysis
Routine investigations such as fasting and post-prandial blood glucose, serum urea/creatinine, urine for routine and microscopic examination, and 24-hour urinalysis for albumin were carried out using Olympus AU-400 (Mishima Olympus Co. Ltd., Shizouka-ken, Japan) autoanalyzer and Star-21 semi-autoanalyzer (SEAC SRL, via di prato, Calenzano-Firenze, Italy). Patients were screened for microalbuminuria with commercially available dipsticks “Micral-test” procured from Roche Diagnostics India Pvt. Ltd., Mumbai-93, India which has a detection limit of 20 mg/L in spot samples. Serum GST activity was measured spectrophotometrically (Shimadzu UV-2450, Shimadzu Corp., Kyoto, Japan) using 1-chloro-2,4-dinitrobenzene as substrate.Citation19 Malondialdehyde (MDA) was estimated by measuring the thiobarbituric acid reactive substances in serum.Citation20
DNA extraction and genotyping
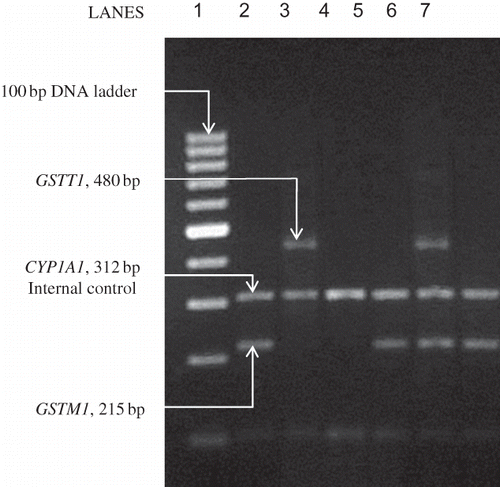
Genomic DNA for genotyping was isolated from ∼500 μL peripheral venous blood collected in EDTA vials using a DNA isolation kit (OmniprepTM, G-Biosciences, St. Louis, MO, USA). A single assay using a multiplex polymerase chain reaction (PCR) was performed for simultaneous gene amplification using the Eppendorf Mastercycler Gradient-5331 (Eppendorf AG, Hamburg, Germany) thermocycler as described by Abdel-Rahman et al.Citation21 Briefly, ∼50 ng of DNA was amplified in a 50 μL multiplex reaction mixture containing 30 pM of each of the following GSTM1 primers (GF – 5′ GAA CTC CCT GAA AAG CTA AAG C 3′ and GR – 5′ GTT GGG CTC AAA TAT ACG GTG G 3′) and of the following GSTT1 primers (TF – 5′ TTC CTT ACT GGT CCT CAC ATC TC 3′ and TR – 5′ TCA CCG GAT CAT GGC CAG CA 3′). As an internal control, the exon 7 of the CYP1A1 gene was also co-amplified (CF – 5′ GAA CTG CCA CTT CAG CTG TCT 3′ and CR – 5′ CAG CTG CAT TTG GAA GTG CTC 3′) in a medium consisting of 1.5 mM MgCl2, 200 μM dNTPs (Bangalore Genei, Bangalore, India), 5 μL 10× PCR buffer (500 mM KCl, 100 mM Tris-HCl, pH 9.0), and 2 U Taq DNA polymerase (New England BioLabs, Beverley, MA, USA). The PCR protocol included an initial denaturation at 94°C for 5 minutes followed by 35 cycles of 2 minutes at 94°C, 1 minute at 59°C, and 1 minute at 72°C followed by a final extension of 10 minutes at 72°C. The final PCR products from co-amplification of GSTM1 (215 bp), GSTT1 (480 bp), and CYP1A1 (312 bp) were visualized after electrophoresis in a ethidium bromide-stained 2% agarose gel on UVP DIGI – DOC IT gel documentation system (UVP, Upland, CA, USA). A sample representative gel picture showing all the possible genotypes is shown in .
Statistical analysis
Demographic data and routine biochemical parameters were compared among the subject groups using one-way analysis of variance (ANOVA) followed by post-hoc Tukey's test. For comparison of the prevalence of different genotypes in different subject groups, the odds ratios were calculated and compared using SPSS software version 17. To analyze the MDA and GST levels among the different genotypic subgroups within the subject groups separately, one-way ANOVA followed by post hoc Tukey's test was used. Analysis of covariance (ANCOVA) was used to compare the homogeneity of the slopes of MDA versus GST in the different groups including their relative interactions in different genotypes.
RESULTS
Demographic data and routine biochemical investigations
The demographic profile with regard to age and sex in subjects of different groups were comparable to each other. Routine biochemical investigations revealed no significant differences among different clinically relevant groups except evidence of poorer blood glucose control among diabetic nephropathy cases compared with diabetics without nephropathy ().
Table 1. Demographic and routine biochemical parameters
Genotyping
On the basis of multiplex PCR analysis of GSTM1 and GSTT1, subjects of the study groups were categorized into four subgroups according to their GST genotypes: GSTM1+/GSTT1+ (no deletions), GSTM1−/GSTT1+ (only GSTM1 deletion), GSTM1+/GSTT1− (only GSTT1 deletion), and GSTM1−/GSTT1− (both deletions). Results of genotyping revealed a prevalence pattern as shown in . Among the different genotypes GSTM1−/GSTT1+ was found to be more or less evenly distributed among all the subject groups, whereas GSTM1+/GSTT1− was appreciably higher among both DM-CKD (26%) and NDM-CKD (36%) groups. GSTM1−/GSTT1− was observed most frequently among DM-CKD patients (32%). Odds ratios were calculated for the presence of CKD with respect to healthy controls as well as diabetics without CKD considering GSTM1 and/or GSTT1 deletions as risk factors as shown in – . It can be observed that the odds for the presence of GSTM1+/GSTT1− and GSTM1−/GSTT1− genotypes were significantly higher in both diabetic and nondiabetic CKD, indicating a strong association between these polymorphisms and CKD. That .this association was not because of the confounding effects of DM in cases of DM-CKD was corroborated by the fact that the odds of the presence of the above-mentioned genotypes were found to be significantly higher in DM-CKD group compared with DM alone.
Table 2(a). Prevalence of different genotypes among the different subject groups and their analysis
Table 2(b). Odds ratios for the presence of different genotypes in nondiabetic CKD compared with healthy controls
Table 2(c). Odds ratio for the presence of different genotypes in diabetic CKD compared with healthy controls
Table 2(d). Odds ratio for the presence of different genotypes in diabetic CKD compared with diabetics without CKD
Genotype–phenotype correlation
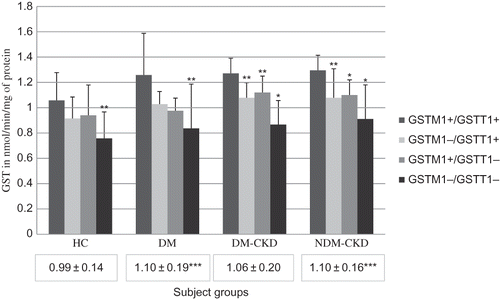
To investigate whether the genotypic variations had any phenotypic implications, we estimated the serum levels of GST enzyme (). Genotypes with one or more deletions were observed to have lower levels of GST compared with GSTM1+/GSTT1+ within the corresponding subject groups. GST levels, in case of concomitant deletion of both the genes, were found to be significantly lower, indicating that double deletion has a negative effect on phenotypic expression. Among patients with DM-CKD and NDM-CKD there was significant decrease in GST levels even in cases with single deletions.
Figure 2. Levels of glutathione S-transferase in different groups.
Bars represent GST values in each genotype. Values within boxes under each group represent mean ± SD for the entire groups. *p < 0.001 between GSTM1+/GSTT1+ and corresponding genotype. **p < 0.05 between GSTM1+/GSTT1+ and corresponding genotype. ***p < 0.05 compared with healthy controls. HC, healthy controls; DM, diabetes mellitus without CKD; DM-CKD, diabetic CKD; NDM-CKD, nondiabetic CKD. GSTM1+/GSTT1+, presence of both the genes; GSTM1+/GSTT1−, presence of GSTM1 and absence of GSTT1; GSTM1−/GSTT1+, absence of GSTM1 and presence of GSTT1; GSTM1−/GSTT1−, absence of both genes.
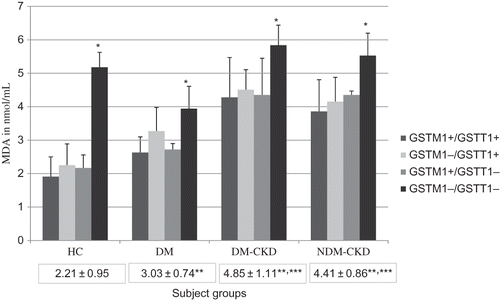
Serum MDA levels were estimated to find out whether genotypic variations and their phenotypic expressions had any bearing on oxidative stress. The MDA levels showed significant elevation in the GSTM1−/GSTT1− group when compared with GSTM1+/GSTT1+ genotype within corresponding subject groups indicating that the double deletion groups have higher oxidative stress ().
Figure 3. Levels of malondialdehyde in different groups.
Bars represent MDA values in each genotype. Values within boxes under each group represent mean ± SD for the entire groups. *p < 0.001 between GSTM1+/GSTT1+ and GSTM1−/GSTT1−. **p < 0.001 compared with healthy controls and ***p < 0.001 compared with DM. HC, healthy controls; DM, diabetes mellitus without CKD; DM-CKD, diabetic CKD; NDM-CKD, nondiabetic CKD. GSTM1+/GSTT1+, presence of both the genes; GSTM1+/GSTT1−, presence of GSTM1 and absence of GSTT1; GSTM1−/GSTT1+, absence of GSTM1 and presence of GSTT1; GSTM1−/GSTT1−, absence of both genes.
To analyze the association of GST expression with oxidative stress, MDA levels were correlated with GST levels among different subject groups with and without deletion of the above-mentioned genes. It was observed that there was significant variation among subjects without these gene deletions when compared with subjects with one or both gene deletions in both healthy and DM subjects () indicating that deletions of GSTT1 and/or GSTM1 genes had an overall negative effect on the expression of the enzyme, that is, there is no increase in GST expression with increasing MDA levels. However, in both diabetic and nondiabetic CKD negative correlation is observed between MDA and GST.
Table 3. Correlation of GST and MDA levels in different subject groups with genotypic variation
DISCUSSION
GST polymorphism and its association with CKD are yet to be well established. Most previous studies concentrated on association of these polymorphisms with DM.Citation9,Citation14,Citation15 In one of our previous studies, we have shown that GSTM1 and GSTT1 double deletion is associated with increased oxidative stress in diabetic nephropathy patients.Citation22
Our study was designed to find out whether the GSTM1 and GSTT1 null polymorphisms are associated with oxidative stress in diabetic and nondiabetic CKD patients. Accordingly, healthy controls and three different groups of patients were recruited: (i) diabetics without CKD (DM), (ii) diabetic CKDs (DM-CKD), and (iii) nondiabetic CKDs (NDM-CKD). The first two groups of patients were included to exclude the confounding effects of DM, because DM is known to be the single largest cause of CKD. The blood samples collected from these patients were analyzed to study the genetic polymorphism and oxidative stress. On the basis of the results of genetic polymorphism, each group was further subdivided into four genotypic subgroups as mentioned in the Results section.
Interestingly, it was observed that GSTT1 null genotype, whether present singly or in combination with GSTM1 null genotype, was significantly associated with CKD irrespective of the presence of diabetes. Most other similar studies reported in the past were conducted on patients with diabetic nephropathy. Interestingly, Yang et al.Citation9 reported GSTT1 null genotype to be a risk factor for diabetic CKD. Recently, Agrawal et al.Citation16 reported that GSTP1-313 allele and null alleles of GSTM1 and GSTT1 are strong predisposing risk factors for ESRD. Unlike our observation, they suggested GSTM1 null allele as risk factor for ESRD. These authors have not clarified whether the recruited ESRD patients also include diabetics, because several studies have reported GSTM1 null genotypes as risk factor for the development of both diabetes and diabetic nephropathy.Citation15,Citation23
We estimated serum levels of GST to study the phenotypic implications of these genetic polymorphisms and found that deletion of one or both of the GST genes were reflected in the significantly lower GST levels. Among the genotypes, the GSTM1−/GSTT1− group had the least GST levels pointing toward the additive effect of these deletions. Our results corroborate with that of Zhong et al.Citation11 who reported reduced erythrocyte GST activity in Chinese population with combined GSTP1 (another polymorphic gene in GST family) and GSTM1 null mutations.
To see whether the decreases in GST level were associated with increased oxidative stress, extent of lipid peroxidation was estimated. We demonstrated that there is a significantly increased MDA level among GSTM1−/GSTT1− genotypes in all subject groups compared with GSTM1+/GSTT1+ genotype. Only one previous study has reported the association of GSTT1 polymorphism with markers of lipid peroxidation in diabetics in relation to coronary heart disease but none is available in relation to CKD.Citation17
To analyze the variation of MDA levels in response to GST polymorphism, we used ANCOVA and found that among the healthy population there is an increase in the expression of GST with increasing oxidative stress among GSTM1+/GSTT1+ genotype compared with other genotypes with one or more gene deletions. This implies that if the genetic machinery is functional then compensatory mechanisms are activated to cope up with increased oxidative stress by increasing the expression of GST. But this pattern was not evident among CKD groups possibly because when kidneys are affected, even the presence of GSTM1+/GSTT1+ genotypes cannot keep pace with increasing oxidative stress, signifying that impairment of kidney function may play a major role in the development of oxidative stress in persons having normal GST activity.
The results of this study suggest that GSTT1 and/or GSTM1 null genotypes are associated with higher oxidative stress in both diabetic and nondiabetic CKD. This could be the underlying mechanism for the observed association of these polymorphisms with CKD and may be considered a possible target for preventive and early intervention strategies in high-risk individuals. Interestingly, GSTT1 deletion was observed to be associated with both diabetic and nondiabetic CKD irrespective of the GSTM1 status. The fact that this study is cross-sectional in design and the sample size in the GSTM1−/GSTT1− group among healthy controls is small limits the population-attributable risk. Hence, these results should be validated by poly-ethnic prospective trials with larger sample size using mRNA expression studies.
Acknowledgments
Author S.K. Datta is grateful to Indian Council of Medical Research, New Delhi, for providing partial funding for this study, which is a part of his MD thesis. The authors are also thankful to the Department of Biostatistics and Medical Informatics, University College of Medical Sciences, Delhi, for its support regarding the statistical aspects.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Zwolinska D, Grzeszczak W, Kilis-Pstrusinska K, Szprynger K, Szczepanska M. Lipid peroxidation and antioxidant enzymes in children with chronic renal failure. Pediatr Nephrol. 2004; 19:888–892.
- Mahajan S, Kalra OP, Tripathi AK, Ahuja G, Kalra V. Phagocytic polymorphonuclear function in patients with progressive uremia and the effect of acute hemolysis. Ren Fail. 2005;27:357–360.
- Dandona P, Thusu K, Cook S, Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445.
- Stanton RC. Diabetic nephropathy and oxidative stress: A report. US Endocr DS. 2006;2:65–67.
- Mannervik B, Awasthi YC, Board PG, Nomenclature for human glutathione transferases. Biochem J. 1992;282:305–306
- Prohaska JR. The glutathione peroxidase activity of glutathione S-transferases. Biochem Biophys Acta. 1980;611: 87–98.
- Galli F, Rovidati S, Benedetti S, Overexpression of erythrocyte glutathione S-transferase in uremia and dialysis. Clin Chem. 1999;45:1781–1788.
- Harrison DJ, Kharbanda R, Cunningham DS, McLellan LI, Hayes JD. Distribution of glutathione S-transferase isoenzymes in human kidney: Basis for possible markers of renal injury. J Clin Pathol. 1989;42:624–628.
- Yang Y, Kao MT, Chang CC, Glutathione S-transferase T1 deletion is a risk factor for developing end-stage renal disease in diabetic patients. Intern J Mol Med. 2004;14:855–859.
- Pemble S, Schroeder KR, Spencer SR, Human glutathione S-transferase theta (GSTT1): cDNA cloning and characterization of a genetic polymorphism. Biochem J 1994;300:271–276.
- Zhong SL, Zhou S-F, Chen X, Relationship between genotype and enzyme activity of glutathione S-transferases M1 and P1 in Chinese. European J Pharm Sci. 2006;28:77–85.
- Mishra DK, Kumar A, Srivastava DS, Mittal RD. Allelic variation of GSTT1, GSTM1 and GSTP1 genes in North Indian population. Asian Pac J Cancer Prev. 2004;5:362–365.
- Singh S, Kumar V, Thakur S, Genetic polymorphism of glutathione S-transferase M1 and T1 in Delhi population of Northern India. Environ Toxicol Pharm. 2009;28:25–29.
- Fujita H, Narita T, Meguro H, et al.. No association of glutathione S-transferase M1 gene polymorphism with diabetic nephropathy in Japanese type 2 diabetic patients. Ren Fail 2000;22:479–486.
- Kim JH, Moon MK, Kim SW, Glutathione S-transferase M1 gene polymorphism is associated with type 2 diabetic nephropathy. J Korean Diabetes Assoc. 2005;29:315–321.
- Agrawal S, Tripathi G, Khan F, Sharma R, Baburaj VP. Relationship between GSTs gene polymorphism and susceptibility to end stage renal disease among North Indians. Ren Fail 2007;29:947–953.
- Hayek T, Stephens JW, Hubbart CS, A common variant in the glutathione S transferase gene is associated with elevated markers of inflammation and lipid peroxidation in subjects with diabetes mellitus. Atherosclerosis 2006;184:404–412.
- Mustafa MD, Pathak R, Ahmed T, Association of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress markers in preterm labor, Clin Biochem. 2010; 43:1124–1128.
- Habig WH, Pabst MJ, Jacoby WB. Glutathione S-transferase: The first step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139.
- Satoh K. Serum lipid peroxide in cerebrospinal disorder determined by a new colorimetric method. Clinica Chemica Acta. 1978;90:37–43.
- Abdel-Rahman SZ, El-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Letters. 1996;107:229–233.
- Datta SK, Kumar V, Ahmed RS, Tripathi AK, Kalra OP, Banerjee BD. Effect of GSTM1 and GSTT1 double deletions in development of oxidative stress in diabetic nephropathy patients. Ind J Biochem Biophys. 2010;47:100–103.
- Yalin S, Hatungil R, Tamer L, Glutathione S-transferase gene polymorphisms in Turkish patients with diabetes mellitus. Cell Biochem Func. 2007;25:509–513.