Abstract
Rationale: Gentamicin (GM) is an effective antibiotic against severe infection but has limitations related to nephrotoxicity. This study investigates whether green tea extract (GTE), an antioxidant, could ameliorate the nephrotoxic effect of GM in uninephrectomized rats. Objectives: The right kidneys of 40 rats were surgically removed and 1 week later the animals were divided into four groups (n = 10). Group 1 served as control, Group 2 as GTE group, Group 3 as GM group, and Group 4 as GM+GTE group. Kidney function, inflammatory cytokine TNF-α, oxidant and antioxidant parameters of renal tissue, as well as histopathological studies were assessed. Main findings: Injecting uninephrectomized rats with GM induced renal dysfunction as shown by significant elevations in serum creatinine and urea. Serum TNF-α and oxidative stress parameters (superoxide anion and lipid peroxides) were also significantly increased. On the contrary, antioxidative parameters [superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH)] were significantly decreased. Histopathological examination of renal tissue illustrated features of degeneration, marked cellular infiltration, tubular dilatation, and varying degrees of necrosis. GTE given to GM rats reduced these nephrotoxicity parameters. Serum creatinine, urea, and TNF-α were almost normalized in the GM+GTE group. The oxidative stress parameters were significantly decreased and the antioxidative parameters were significantly increased. Conclusion: GTE ameliorates GM-induced nephrotoxicity and oxidative damage by improving antioxidant defense and tissue integrity. Further human studies are necessary to demonstrate the antioxidant effects of GTE on renal diseases. Nevertheless, green tea (GT) may offer an inexpensive, nontoxic, and effective intervention strategy in subjects with a risk for GM-induced nephrotoxicity.
INTRODUCTION
Renal failure is common and one of the single most expensive disorders on a per-patient basis.Citation1 Gentamicin (GM) is a widely used antibiotic that exhibits broad spectrum activity and is particularly valuable in the treatment of severe sepsis. Its use, however, has been restricted because of the chance of nephrotoxicity.Citation2 Otherwise, GM continues to play a valuable role in clinical medicine because of its low cost and its efficacy against Gram-negative and resistant β-lactam microorganisms.Citation3 Nephrotoxicity is due to the selective accumulation of GM in the renal cortex, inhibiting protein synthesis in renal cells and leading to necrosis of cells in the proximal tubules; this results in acute tubular necrosis and eventually acute renal failure.Citation4 There is evidence that reactive oxygen species (ROS) are involved in renal damage.Citation5 Consequently, administration of several compounds with antioxidant activity may have the capacity to protect the kidney against the deleterious effects of drugs known to enhance the generation of ROS like GM.Citation6
Green tea (GT) is widely consumed in the eastern world. It possesses diverse pharmacological properties that include antioxidative, anti-inflammatory, anticarcinogenic, antiarteriosclerotic, and antibacterial effects.Citation7,Citation8 GT polyphenols have shown strong chemopreventive and chemotherapeutic effects against various pathologies.Citation9 Administration of green tea extract (GTE) prevents the generation of thiobarbituric acid reactive substances (TBARS) and significantly attenuates renal dysfunction induced by GM as assessed by the measures of serum creatinine, blood urea nitrogen (BUN), uric acid, and activity of antioxidant enzymes in the kidney homogenate.Citation10 However, until now, the ameliorative effect of GTE against GM-induced nephrotoxicity has not been fully investigated.
Based on the reported antioxidant properties of polyphenolic compounds that are present in the GTE, the hypothesis was made that GTE could ameliorate GM-induced oxidative stress and renal damage. Therefore, the aim of this study was to investigate the protective effect of GTE against GM-induced nephrotoxicity in uninephrectomized rats through the assessment of kidney biochemical parameters such as serum urea, creatinine, cytokine TNF-α, oxidant and antioxidant parameters of renal tissue, as well as histopathological examination of kidney tissues.
MATERIALS AND METHODS
Animals
Forty adult male Wistar rats of similar age (5 months) and weight (170 ± 10 g) were selected for this study. The animals were obtained from the Egyptian Organization of Biological Products and Vaccines (Helwan, Egypt). Animals were housed in stainless steel wire-mesh suspended rodent cages under environmentally controlled conditions. The ambient temperature was 25 ± 2°C and the light/dark cycle was 12/12 h. Rodent chew diet and water were allowed ad libitum. All animals received human care in compliance with the guidelines of the Ethical Committee of Medical Research of National Research Centre, Egypt.
Experimental design
Two weeks after acclimatization, all 40 rats were anesthetized by intraperitoneal injection with sodium pentobarbital (50 mg/kg).Citation11 The right kidneys of 40 rats were surgically removed and were administered a prophylactic dose of penicillin (4000 IU/kg IM). One week later, the animals were divided into four groups of 10 animals each. The animals of Group 1 served as control. Group 2 received an oral administration of 300 mg/kg/day GTE tablets (750 mg GTE/tablet; EL Obour Pharma for techno made group, El Obour city Egypt) continuously for 30 days using an intragastric enteral feeding protocolCitation12 and served as the GTE group. Group 3 was injected subcutaneously with GM (Sigma chemical, St. Louis, MO, USA) 50 mg/kg twice daily for 15 consecutive daysCitation13 and served as the GM group. Group 4 was injected with GM subcutaneously 50 mg/kg twice daily for 15 days and orally administered GTE 300 mg/kg/day for 15 days concomitantly with GM and 15 days after and served as GTE+GM group.
Sampling
At the end of the treatment period (30 days), animals were fasted overnight and then anesthetized using light diethyl ether.Citation14
Blood sampling
Serum samples were obtained through retro-orbital bleeding. Blood was collected in a dry centrifuge tube for serum separation, centrifuged at 3000 × g for 15 min (4°C), and stored at 20°C as aliquots for further determinations of creatinine, urea, and TNF-α.
Tissue sampling
After blood collection, all animals were rapidly sacrificed and the left kidneys of the rats were removed by dissection and washed with 0.9% NaCl. Part of the harvested organs were rapidly frozen in liquid nitrogen (−170°C) then stored at −20°C for further determinations of: superoxide anion (O2), susceptibility for lipid peroxidation, superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH). The other parts of the kidneys were stored in 10% formalin saline at 4°C then processed for histopathological study.
Biochemical analysis
Creatinine concentration was measured as described by HenryCitation15 using Diamond Diagnostic Kits (Cairo, Egypt), following the instructions of the manufacturer. Serum urea was quantitatively determined using the QCA Kit (Urea, Quimica Clinica Apilicada S.A, Tarragona, Spain) with the modified Searcy method.Citation16 Serum TNF-α was determined according to Chen et al.Citation17 by solid-phase ELISA using rat TNF-α Kits (Biosource International, California, USA) and microtiter plate reader (Fisher Biotech, Offenburg, Germany).
Tissue superoxide anion was determined according to the modified method of Hassoun and Stohs,Citation18 following the principle of Babior et al.Citation19 Principle: Superoxide anion production in renal tissue was measured on the basis of reduction of cytochrome c.
Lipid peroxidation was determined according to Placer et al.Citation20 Principle: Measurement of lipid peroxidation in renal tissue depends on the determination of TBARS content.
Total protein was determined according to Chromy and FischerCitation21 using the diagnostic kits provided by Biocon (Mecklenburg-Vorpommem, Germany), following the manufacturer's instructions. The activity of renal tissue SOD was determined according to Marklund.Citation22
Tissue CAT activity was determined according to Clairbrone.Citation23 The method is based on the decomposition of hydrogen peroxide by CAT enzyme; such a decomposition rate is directly proportional to the activity of CAT enzyme. GSH was determined using Ellman's reagent as modified by Ahmed et al.Citation24
Histological studies were done according to Prophet et al.Citation25 At the end of the experimental period, the left kidney of each animal was removed. Small pieces of fresh tissue were fixed in 10% neutral formalin and processed for histological study. Paraffin sections of 4-μm thick were stained with hematoxylin and eosin. The histopathological changes were evaluated in several sections from each group using a light microscope.
Using the SPSS computer program, the obtained data were statistically analyzed according to Steel and Torrie.Citation26 The results were presented as mean ± SD. The differences between the mean values were determined by analysis of variance (ANOVA), followed by Duncan's multiple rank testCitation27 using the MSTATC computer program. Means with different letters are significantly different.
RESULTS
Blood fractions
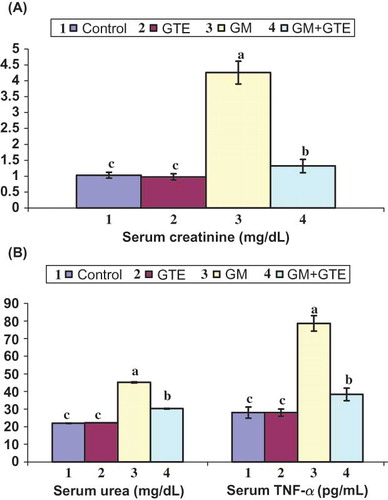
Injecting uninephrectomized rats with GM 50 mg/kg twice daily for 15 consecutive days caused a marked increase in the level of serum creatinine to 4.26 ± 0.36 mg/dL (A). There was a considerable increase in BUN to 45.16 ± 4.32 mg/dL after GM injection. On the contrary, the GTE group demonstrated normal kidney functioning in both biochemical (creatinine and BUN) parameters. Moreover, in the GM+GTE group, creatinine leveled at 1.32 ± 0.21 after 30 days of GTE administration. The urea in the same group also normalized to 30.24 ± 3.54 mg/dL. Injection of uninephrectomized rats with GM induced significant increase in serum TNF-α level (B). This increase was approximately 2.8-fold in comparison with the control or GTE group. Administration of GTE in Group 4 (GM+GTE) significantly attenuated serum TNF-α levels as compared with the GM group (about 1.4 of the control).
Renal tissue fractions
Injection of uninephrectomized rats with GM significantly increased renal tissue superoxide anion level as compared to the control and GTE group, by approximately 3.4- and 3.3-fold, respectively (). However, administration of GTE in combination with GM normalized the level of renal tissue superoxide anion to 25.32 ± 2.14 nmol cytochrome reduced/min/g tissue. Induction of nephrotoxicity by GM in Group 3 significantly elevated the renal tissue lipid peroxide level by 5.2- and 5.3-fold in the GTE group. Coadministration of GTE with GM in Group 4 normalized the level of renal tissue lipid peroxides to 28.72 ± 2.92 nmol MDA/g tissue.
Figure 2. Effect of GTE on renal tissue fractions of uninephrectomized rats injected with 50 mg/kg GM twice daily for 15 days. Graphs of each parameter with the same alphabetical letter (a, b, c) are not significantly different at p < 0.
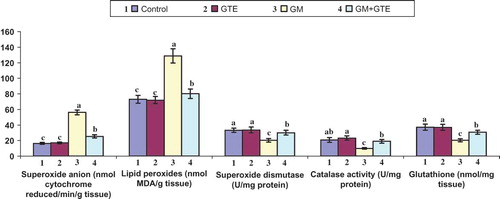
Treating uninephrectomized rats with GM in Group 3 significantly lowered renal tissue SOD by 27% and 28% as compared to the control group and the GTE group, respectively. Coadministration of GTE with GM in Group 4 resulted in a significant elevation in renal tissue SOD, CAT, and glutathione activities. Histopathological examination of renal tissue revealed a renoprotective effect of GTE and showed stromal cellular infiltration and decreased amounts of necrosis.
Histopathological examination of renal tissue from rats receiving 50 mg/kg GM twice daily for 15 days revealed features of degeneration, extensive hemorrhage, focal fibrosis, necrotic changes of renal tissue, and round cell infiltration (C). However, coadministration of GTE with GM documented a renoprotective effect of GTE and showed only mild infiltration of round cells among tubules (D). Treatment with GTE in Group 2 revealed normal renal tissues (B), similar to the control group (A).
Figure 3. Effect of GTE on the histopathological changes of renal tissue in uninephrectomized rats injected with GM. (A) Renal tissue of control rats showing normal renal parenchyma [hematoxylin and eosin (H&E) × 1200]. (B) Renal tissue of rats received 300 mg/kg/day GTE for 30 days showing normal renal tissue (H&E × 600). (C) Renal tissue of rats received 50 mg/kg GM twice daily for 15 days showing extensive hemorrhage, focal fibrosis, necrotic changes of renal tissue, and cell infiltration (H&E × 800). (D) Renal tissue of rats received 50 mg/kg GM twice daily for 15 days and 300 mg/kg/day GTE for 30 days showing mild infiltration of round cells among tubules (H&E × 800).
![Figure 3. Effect of GTE on the histopathological changes of renal tissue in uninephrectomized rats injected with GM. (A) Renal tissue of control rats showing normal renal parenchyma [hematoxylin and eosin (H&E) × 1200]. (B) Renal tissue of rats received 300 mg/kg/day GTE for 30 days showing normal renal tissue (H&E × 600). (C) Renal tissue of rats received 50 mg/kg GM twice daily for 15 days showing extensive hemorrhage, focal fibrosis, necrotic changes of renal tissue, and cell infiltration (H&E × 800). (D) Renal tissue of rats received 50 mg/kg GM twice daily for 15 days and 300 mg/kg/day GTE for 30 days showing mild infiltration of round cells among tubules (H&E × 800).](/cms/asset/cea7204f-6714-40f3-8ec1-62e26a3a9617/irnf_a_517350_f0003_b.jpg)
DISCUSSION
In the control and GTE groups, rats demonstrated normal renal functions (normal serum creatinine and urea). Histopathological sections of the same two groups also reflected normal renal structure.
The toxic renal effects of GM in Group 3 were manifested by a marked increase in the levels of serum creatinine and BUN. Similar degrees of GM toxicity have been reported by Soliman et al.Citation28 and Khan et al.Citation9
GM-induced nephrotoxicity occurs because of the interaction between molecular and subcellular components (lysosomes and mitochondria), where GM binds to the anionic phospholipids of cell membranes and gains access to the cell interior before binding to the subcellular organelles or being taken up by lysosomes. Accumulation of GM within the renal cortex is intimately related to the pathogenesis of renal failure.Citation29
GM injections significantly elevated serum levels of TNF-α. The inflammatory cytokine TNF-α itself causes injury to renal epithelial cells because it stimulates the production of ROS and produces oxidative stress by sensitizing infiltrating leukocytes.Citation30 TNF-α appears to be involved in many types of inflammatory processes and has been negatively correlated with SOD, CAT, and renal tissue GSH.Citation31
These results reveal that GM-induced nephrotoxicity generates ROS. This is manifested by a significant increase in renal tissue superoxide anion and MDA production and a significant reduction in GSH, CAT, and SOD activities. These results are in agreement with the reports by Atessahin et al.Citation32 The generation of ROS by GM is attributed to renal cortical mitochondrial injury.Citation33 Administration of SOD and Vitamin E reduced nephrotoxicity.Citation34
A number of pathways exist by which reduction in glutathione concentrations may be involved with the generation of reactive oxygen radicals or organic peroxidases using GSH as an electron donor generating oxidized glutathione (GSSG), which can be reversed by NADPH-dependent glutathione reductase. This may in turn lead to the depletion of intracellular GSH resulting in cellular injury.Citation35
The biochemical analysis of this study is confirmed by the histopathological examination of the renal tissue, where GM groups demonstrated extensive hemorrhage, focal fibrosis, necrotic changes, and infiltration (). These results are in accordance with the findings reported by Soliman et al.Citation28 However, coadministration of GTE with GM in Group 4 revealed renoprotective effects and showed only mild infiltration (). The renoprotective effect of GTE has been previously reported by Arteel et al.Citation12 and Khan et al.Citation9
It has been suggested that oxidative stress may contribute to the progression of renal damage,Citation36 hence antioxidants may provide protection against disease onset or progression. GTE polyphenols possess antioxidant properties and appear to be potent scavengers of hydroxyl radicals (OH−), known for their damaging effects on cellular macromolecules.Citation37 Takabayashi et al.Citation38 showed that a single dose of GTE significantly decreased the amount of 8-hydroxyguanosine, a marker of OH− attack on DNA.
The mechanism for GTE protection is not clear but may be mediated by its antiproliferative components, or polyphenols – known as flavonols or catechins. These polyphenols include epicatechin, epicatechin-3-O-gallate, epigallocatechin, gallocatechin-3-O-gallate, and epigallocatechin-3-O-gallate with the latter being the main polyphenol in GT, the protector against renal injury.Citation39
The protective effects of GTE are manifested by the considerable reduction of serum creatinine and BUN levels where the main polyphenols (EGCG) in GT have been hypothesized to participate in the elimination of uremic toxins and prevention of renal disorders.Citation40 GT polyphenols inhibit the synthesis of potent vasoconstrictors such as thromboxane A2 (TxA2) and prostaglandin D2 (PGD2) generation.Citation41
The hypothesis that GTE exhibits anti-inflammatory properties has been supported by the findings that intake of GTE significantly reduced serum TNF-α levels. Through this connection, Yang et al.Citation42 suggested GT polyphenols reduce inflammatory responses by attenuating NF-κB activation. The activation of NF-κB leads to an increase in expression of many genes whose products mediate immune responses. These include proinflammatory cytokine TNF-α and adhesion molecules. In addition, GTE has powerful antioxidant properties: it significantly elevates the bioactivities of SOD, GSH, and CAT while significantly decreasing superoxide anion and lipid peroxide levels in renal tissues. GTE polyphenols act as antioxidants by scavenging ROS and nitrogen species and chelating redox-active transition metal ion. GTE polyphenols also function indirectly as antioxidant through: (1) inhibition of pro-oxidants as inducible nitric oxide synthase, lipooxygenase, cyclooxygenase, and xanthine oxidase; (2) inhibition of redox-sensitive transcription factors, NF-κB, activator protein-1, and SOD.Citation43
Histopathological examination of renal tissue revealed the renoprotective effect of GTE and showed stromal cellular infiltration and decreased the numbers of necrotic cells; as reported by Mohamadin et al.Citation10
In conclusion, GTE ameliorates GM-elicited nephrotoxicity and oxidative damage by improving antioxidant defense and tissue integrity. Further studies in both animals and humans are necessary to demonstrate the antioxidant properties and other effects of GTE on renal diseases. Nevertheless, GT may offer an inexpensive, nontoxic, and effective intervention strategy in patients prone to nephrotoxicity.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- USRDS. Incidence and prevalence of ESRD: In Annual Data Report. Bethesda: The national institutes of health, National Institutes of Diabetes and Digestive and Kidney Deseases; 1998:23–25.
- Appel GB, Neu HC. The nephrotoxicity of antimicrobial agents. N Engl J Med. 1997;269:663–670.
- Edson RS, Terell GL. The aminoglycosides. Mayo Clin Proc. 1999;74:519–528.
- Sundin DP, Sandoval R, Molitoris BA. Gentamicin inhibits renal protein and phospholipid metabolism in rats. J Am Soc Nephrol. 2001;12:114–123.
- Martinez-Salgado C, Eleno N, Lavares P. Involvement of reactive oxygen species on gentamicin induced mesangial cell activation. Kidney Int. 2002;62:1082–1092.
- Abdel Raheem IT, El Sherbiny GA, Taye A. Green tea ameliorates renal oxidative damage induced by gentamicin in rats. Pak J Pharm Sci. 2010;23(1):21–28.
- Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea. J Altern Complement Med. 2005;11:639–652.
- Zhao P. The health of tea polyphenols and their antioxidant mechanism. J Clin Biochem Nutr. 2006;38:59–68.
- Khan SA, Priyambada S, Farooq N, Khan S, Khan W, Yusufi AN. Protective effect of green tea extract on gentamicin induced hepatotoxicity and oxidative damage in rat kidney. Pharmacol Res. 2009;51(1):51–57.
- Mohamadin AM, El Bahbishy HA, El Mahdy MA. GTE attenuates cyclosporine A induced oxidative stress in rats. Pharmacol Res. 2005;51(1):51–57.
- Tomita H, Sogabe H, Nakazato S, Monoclonal antibody 1-22-3-induced glomerulonephritis in uninephrectomized rats as a model of progressive renal failure. Nephrol Dial Transplant. 2005;20:2358–2367.
- Arteel GE, Uesugi T, Bevan LN, GTE protects against early alcolhol induced liver injury in rats. Biol Chem. 2002; 383(3–4):663–770.
- Buyukaf K, Yazar AZ, Dumez D, Ozturk H, Polat G, Levent A. Effect of trapidil, an antiplatelet and vasodilator agent on gentamicin induced nephrotoxicity. Pharmacol Res. 2001; 44(4):321–328.
- Van Herck H, Baumans V, Brandt JWM, Orbital sinus blood samplingin rats as performed by different animal technicians: Then influence of technique and expertise. Lab Anim. 1996;32:377–386.
- Henry RJ. Clinical Chemistry, Principles and Techniques. 2nd ed. New York: Harper and Row; 1974.
- Searcy RI, Reardon JE, Forenam JA. Enzymatic urea kits. Am J Med Tech. 1967;33:15–20.
- Chen W, Jin W, Cook M, Weiner HL, Wahi SM. Oral delivery of group A streptococcal cell walls augments circulating TGF, beta and suppresses streptococcal cell wall arthritis. J Immunol. 1998;161(11):6297–6304.
- Hassoun EA, Stohs SJ. Cadmium induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J 744 A-1 cultures. Toxicology. 1996;112(3):219–230.
- Babior BM, Kipnes RS, Curnutte ST. Biological defense mechanism, the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52(3): 741–744.
- Placer ZA, Cushman LL, Johnson BC. Estimation of lipid peroxidation (malondialdehyde) in biochemical systems. Anal Biochem. 1966;16:359–365.
- Chromy N, Fischer J. Photometric determination of total protein in lipemic sera. Clin Chem. 1977;23(4):754–756.
- Marklund SL. Pyrogallol autooxidation. In: Greenwold RA, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985:243–247.
- Clairbrone A. Catalase activity. In: Greenwold RA, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985:283–284.
- Ahmed AE, Gamal IH, Loh J, Abdel Rahman SZ. Studies on mechanism of haloacetonitrile induced gastrointestinal toxicity interaction of dibromoacetonitrile with glutathione S-transferase in rats. J Biol Toxicol. 1991;612:115–121.
- Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory Methods in Histotechnology. Washington, DC: American Registry of Pathology; 1992.
- Steel RDG, Torrie HJ. Principles and Procedures of Statistics: A Biometrical Approaches, 2nd ed., 4th Print Singapore: Mc Graw Hill; 1984.
- Duncan DB. Multiple range test and multiple F test. Biometrics. 1955;1:1–42.
- Soliman KM, Hamid M, Othman AI. Effect of carnosine on gentamicin induced nephrotoxicity. Med Sci Monit. 2007; 13(3):73–83.
- Erdem A, Gundogan NU, Usubutun A, The protective effect of taurin against gentamicin induced acute tubular necrosis in rats. Nephrol Dial Transplant. 2000;15:1175–1182.
- Ramesh G, Reaves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110(6):835–842.
- Akbulut G, Kilek ON, Kahraman A, Koken T, Serteser M. The correlation between tissue renal oxidative stress parameters and TNF-α levels in an experimental model of ischemia reperfusion injury in mice. Ulus Travma Derg. 2005;11(1):11–16.
- Atessahin A, Karahan I, Yilmaz S, Ceribasi AO, Princci I. The effect of manganese chloride on gentamicin induced nephrotoxicity in rats. Pharmacol Res. 2003;48(6):367–642.
- Baliga R, Ueda N, Waiker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–997.
- Kumar KV, Naidu MUR, Shifow AA, Ratnakar KS. Probucol protects against gentamicin induced nephrotoxicity in rats. Ind J Pharmacol. 2000;32:108–113.
- Rush GK, Smith JH, Newton JE, Hook JB. Chemically induced nephrotoxicity role of metabolic activation. CRC Crit Rev Toxicol. 1986;13:99–160.
- Quiroz Y, Ferrebuz A, Roero F, Vaziri ND, Todriguez-Iturbe B. Melatonin ameliorates oxidative stress, inflammation, proteinuria and progression of renal damage in rats with renal mass reduction. Am J Physiol Renal Physiol. 2007; 294:336–344.
- Yoshioda H, Kurosaki H, Yoshinaga K, Saito K. Beta ray induced scission of DNA in tritiated water and protection by a green tea percolate and (–) epigallocatechin gallate. Biosci Biotecnol Biochem. 1997;61:1560–1563.
- Takabayashi F, Harada N, Tahara S, Kaneko T, Hara Y. Effect of green tea catechins on the amount of 8-hydroxydeoxyguanosine (8 OHdG) in pancreatic and hepatic DNA after a single administration of N-nitrosobis (2-oxopropyl) amine (BOP). Pancreas. 1997;15:109–112.
- Shin BC, Ryu HH, Chung JH, Lee BR, Kim HL. The protective effects of green tea extract against L-arginine toxicity to cultured human mesangial cells. J Korean Med Sci. 2009; 24(Suppl.): 204–209.
- Jung YD, Kim MS, Shin BA, EGCG, a major component of green tea inhibits tumor growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001; 84:844–850.
- Son DJ, Cho MR, Jin YR, Antiplatelet effect of green tea catechins: A possible mechanism though arachidonic acid pathway. Prostaglandins Leukot Essent Fatty Acids. 2004; 1(1):25–31.
- Yang F, De Villiers WJ, McClain CJ, Varilck GW. Green tea polyphenols block endotoxin induced tumor necrosis factor production and lethality in a murine model. J Nutr. 1998; 128(12):2334–2340.
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: Evidence for nutritional sciences. J Nutr. 2003;133: 3275–3284.