Abstract
Degradation of extracellular matrix (ECM) by enhanced production of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in diabetes leads to nephropathy. Cyclooxygenases (COX) further increase levels of these MMPs. The objective of present study was to inhibit MMP-2 and MMP-9 by combination of minocycline and aspirin to treat diabetic nephropathy. Diabetes was induced in male Wistar rats by streptozotocin (STZ, 55 mg/kg i.p.). Four weeks after diabetes induction, the rats were treated with minocycline (50 mg/kg, p.o.), aspirin (50 mg/kg, p.o.), or minocycline (50 mg/kg, p.o.) plus aspirin (50 mg/kg, p.o.) for a period of 4 weeks. At the end of eighth week fluid input, urine output, and renal function tests were carried out for diagnosis of diabetic nephropathy. Renal hypertrophy was measured and histopathology was done to evaluate renal damage. Diabetes produced significant loss of body weight, polyuria, polydipsia, hyperglycemia, and increase in blood pressure. Serum creatinine, urea, and blood urea nitrogen levels were found to be increased significantly in the STZ group diabetic rats. Treatment with combination of minocycline and aspirin significantly prevented the rise in creatinine, urea, and blood urea nitrogen levels and increased creatinine clearance. Image analysis of kidneys revealed that collagen level was significantly decreased in combined treated group when compared with control. Results of present study suggest that MMP-2 and MMP-9 inhibition in presence of COX inhibitor prevents the development of experimental diabetic nephropathy in rats and can be a potential approach for the treatment.
INTRODUCTION
More than 30% of diabetes mellitus (DM) patients develop clinically evident diabetic nephropathy 10–20 years after the onset of diabetesCitation1 and diabetic nephropathy is now the most common cause of end-stage renal disease requiring renal transplantation or chronic hemodialysis.Citation2 Despite the benefits derived from the current therapeutics for diabetic nephropathy, mainly strict control of glucose, blood pressure, and blockade of the renin–angiotensin system, these strategies still provide imperfect protection against renal progression.Citation3,Citation4 This imperfection points to the need for newer therapeutic strategies that have potential to affect primary mechanisms contributing to the pathogenesis of diabetic nephropathy.Citation5 These include increased oxidative stress, renal polyol formation, advanced glycated end-products accumulation, and presclerotic cytokines such as transforming growth factor beta-1 (TGF-β1). These pathways ultimately lead to increased renal albumin permeability and extracellular matrix (ECM) accumulation, which result in increasing proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis.
The accumulation of ECM within the kidney is an ultrastructural hallmark of diabetic nephropathy and is directly linked to a decline in renal function.Citation6 This increase of ECM can result from either increased synthesis and decreased degradative activity or both. Several studies have shown that high glucose concentration increases synthesis of ECM proteins in both mesangial and tubular epithelial cells.Citation7 Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidase that mediate the degradation or remodeling of the ECM.Citation8 The ECM is a multifunctional complex of proteins and proteoglycans assembled in a highly organized manner that contributes to the structural integrity of cells and tissue within an organ system.Citation9 The basement membrane which provides structural support to the vasculature is composed of ECM molecules such as type IV collagen, laminin, and fibronectin.Citation10 Various factors are involved in maintaining the integrity of the ECM and the tissues it supports. However, in certain pathological circumstances the ECM is modulated such that the structure of the tissues becomes damaged or destroyed.Citation11,Citation12 Two members of the MMP family, matrix metalloproteinase-2 (MMP-2, 72 kD gelatinase/gelatinase A) and matrix metalloproteinase-9 (MMP-9, 92 kD gelatinase/gelatinase B), degrade the ECM components of the basement membrane. Their substrates include types IV and V collagen, fibronectin, elastin, and denatured interstitial collagens.Citation13 Two groups of extracellular proteinases that have been shown to play a role in the renal revascularization seen in diabetic nephropathy are the MMP-2 and MMP-9. Plasmin in turn degrades components of the matrix and can be involved in the activation of the latent MMPs.
We hypothesized that remodeling of ECM could be attenuated by minocycline-induced inhibition of MMP-2 and MMP-9 and further it can be potentiated by aspirin because of its Cyclooxygenases (COX) and tissue plasminogen activator (tPA) inhibitory action. The basis for this hypothesis was a case report by AhujaCitation14 on an incidental reduction of glomerulonephritis proteinuria by 70% with doxycycline treatment. Earlier, we have reported attenuation of diabetic neuropathyCitation15 and diabetic retinopathyCitation16 by this combination in rats. In the present study, we have targeted MMP-2 and MMP-9 over-activation in diabetic nephropathy using a known MMP-2 and MMP-9 inhibitor, minocycline with a nonselective COX inhibitor aspirin. Although aspirin is nonselective COX inhibitor, it has been selected because of its inhibitory effect on tPA. This finding may indicate the potential benefit of minocycline along with aspirin treatment in easing the chronic consequences of diabetic nephropathy.
MATERIALS AND METHODS
Chemicals and Drug Solution Preparation
Streptozotocin (STZ) was purchased from Sigma (St. Louis, MO, USA). Minocycline was procured from US Vitamins, Mumbai, India, as a gift sample. Aspirin was purchased from Central Drug House, Mumbai, India. Glucose oxidase–peroxidase (GOD/POD) glucose kit was purchased from Erba Diagnostics, Mumbai, India. All other chemicals were purchased from Merck (Mumbai, India). Minocycline was dissolved in distilled water. Aspirin was suspended in aqueous solution of 0.5% carboxymethyl cellulose. STZ was freshly dissolved in ice-cold citrate buffer (pH 4.5) solution.
Animals
Male Wistar rats (210–250 g) were purchased from the Haffkine Institute (Mumbai, India) and were housed at a temperature of 25 ± 1°C and relative humidity of 45–55% in a clean environment under 12:12 h light and dark cycle. The animals had free access to food pellets and filtered water was made available ad libitum. The research protocol was approved by Institutional Ethical Committee (IEC) of School of Pharmacy and Technology Management, NMIMS University, Mumbai, constituted under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Induction of Diabetes
A single dose (55 mg/kg, i.p.) of STZ was used for induction of diabetes in rats. Age-matched control rats received the equal volume of vehicle. Diabetes was confirmed after 48 h of STZ injection and again on weekly basis during the experiment. Plasma glucose levels were estimated using GOD/POD kit and rats with plasma glucose level >350 mg/dL were considered for further studies.
Experimental Design and Drug Treatment
Four weeks after the diabetic induction, treatments were given for further 4 weeks (fifth, sixth, seventh, and eighth weeks). After 4 weeks, normoglycemic and diabetic rats were randomly divided into experimental groups and treated with drugs as follows:
Diabetic vehicle treated Group 1 (DB + DW) was treated with distilled water 4 mL/kg, p.o.).
Diabetic vehicle treated Group 2 (DB + 0.5% CMC) was treated with 0.5% carboxymethyl cellulose solution (1 mL/kg, p.o.).
Diabetic Group 3 (DB + MINO) was treated with minocycline alone (MINO, 50 mg/kg, p.o.).
Diabetic Group 4 (DB + ASP) was treated with aspirin alone (ASP, 50 mg/kg, p.o.).
Diabetic Group 5 (DB + MINO + ASP) was treated with combination of minocycline (MINO, 50 mg/kg, p.o.) and aspirin (ASP, 50 mg/kg, p.o.).
Age-matched Normal Group 6 was untreated.
Renal Function Tests
At the end of the eighth week, rats were kept individually in metabolic cages for 24 h to collect urine for the measurement of urine output and renal function. Renal function was assessed by measuring plasma and urine levels of creatinine, urea, and urine albumin excretion using semi-auto-analyzer (Erba Chem-5 plus; Transasia, Mumbai, India). Creatinine clearance was measured as an index of glomerular filtration rate (GFR).
Systemic Blood Pressure
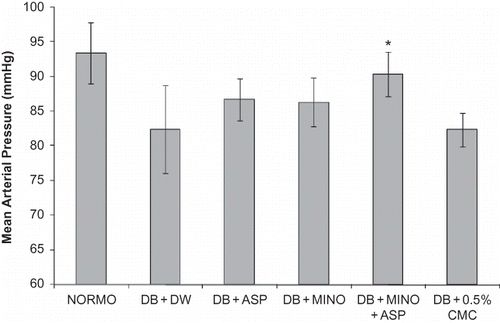
Eight weeks after STZ or vehicle, rats were anesthetized with pentobarbital sodium and body temperature was maintained at 37 ± 1°C. Systemic blood pressure was monitored via a catheter inserted into the femoral artery. Blood pressure was recorded digitally through a data acquisition system (Power Lab system, AD Instruments, New South Wales, Australia). Mean arterial pressure (MAP) was calculated using the following formula: MAP = diastolic pressure + 1/3 (systolic pressure – diastolic pressure).
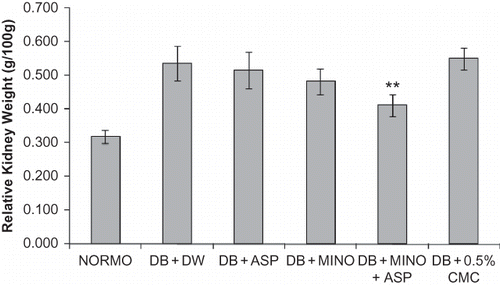
Renal Hypertrophy and Histopathology
After completion of MAP recording, rats were perfused via the abdominal aorta with 0.1 mol/L PBS (150 mL [pH 7.4], 180–220 mmHg) for 1–2 min to remove circulating blood. The right kidney was then removed, weighed, and kidney/body weight ratio calculated. Data were expressed as relative organ weight of one kidney to 100 g of total body weight. Paraformaldehyde-fixed (4%) tissue was embedded in paraffin and sectioned at 5 μm for morphologic analysis. The kidney specimens were fixed in 4% paraformaldehyde solution and embedded in paraffin. Sections were cut at 4 mm with a microtome and deparaffined with xylene. They were stained with Masson's trichrome stain and hematoxylin and eosin (H&E) using standard histological procedure. Stained kidney sections were observed under a light microscope.
Index of Tubulointerstitial Fibrosis
Masson's trichrome-stained sections were examined and the degree of tubulointerstitial fibrosis determined using a semiquantitative scoring method: 0 = no lesions showing cell infiltration and fibrosis, 1 = minimal injury (single focus of lesion), 2 = mild injury (more than two isolated foci), 3 = moderate injury (more than five isolated foci), and 4 = severe injury (more than 10 isolated foci or diffuse infiltration and fibrosis).
Histology of Renal Artery
Paraffin-embedded cross-sections (5 μm thick) of renal artery were stained with hematoxylin and eosin (H&E) solution (Sigma), and Elastin Van-Giesen staining was performed using the Elastic Stain kit to stain collagen fibers. Six sections of artery from all animals were used for collagen fiber analysis. Images were captured using a CCD camera mounted in a microscope (Motic®). Area of red-colored collagen was measured using Scion Image software (NIH, Bethesda, Maryland, USA). Area of collagen was represented as percent value of total area measured.
SDS-PAGE Zymography
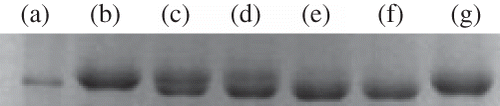
Rats from each group were anesthetized deeply and then perfused transcardially with ice-cold PBS, pH 7.4. The left kidneys were removed quickly and frozen immediately on dry ice, and stored at –80°C. Tissue samples were homogenized, centrifuged, and supernatant was collected. Prepared protein samples were loaded and separated by 10% Tris-glycine gel. After separation by electrophoresis, the gel was stained with 0.5% Coomassie Blue R-250 for 30 min and then destained appropriately. MMP-2 (72 kD) and MMP-9 (92 kD) bands were confirmed by comparison with commercially available molecular weight marker.
Statistical Analysis
Data are expressed as mean ± SD. Statistical analysis was performed using Graph Pad Prism (version 4.0, Graph Pad Inc., San Diego, CA, USA) software. For multiple comparisons, one-way analysis of variance (ANOVA) was used. In case ANOVA shows significant differences, post hoc analysis was performed with Dunnet post hoc test; p < 0.05 was considered statistically significant.
RESULTS
Plasma Glucose Levels and Body Weight
STZ-induced diabetic rats showed approximately fivefold increase in the blood glucose levels after STZ administration. Out of 40 animals, 4 animals were discarded from the study due to less increase in BSL (i.e., less than 350 mg/dL). Rest of the 36 animals showed elevated BSL which was consistent throughout the study period (). Diabetic rats showed significant decrease in body weight as compared to age-matched normoglycemic rats (). Treatment with MINO, ASP or combination of MINO and ASP did not produce any change in plasma glucose levels ().
Table 1. Effects of 4-week treatment with MINO, ASP, and MINO in combination with ASP on renal function tests
Renal Function Tests
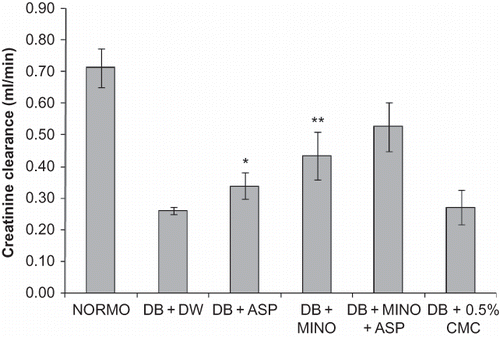
Diabetic rats exhibited marked polyuria, increased urinary albumin excretion, high serum creatinine, as well as blood urea nitrogen. Chronic treatment with MINO + ASP significantly reduced diabetic proteinuria and polyuria, and increased serum creatinine and blood urea nitrogen (). Creatinine clearance was also significantly improved following the administration of MINO + ASP to diabetic rats compared with untreated diabetic rats (). Marked renal hypertrophy was observed in diabetic group. Kidney to body weight/100 g ratio was significantly higher in diabetic group (0.54 ± 0.05, p < 0.05) compared to normoglycemic group (0.32 ± 0.02). Treatment with MINO + ASP attenuated renal hypertrophy significantly when compared with diabetic group (0.41 ± 0.03, p < 0.05, ). Mean arterial pressure was decreased in diabetic group (82 ± 6.4 mmHg) compared to normoglycemic (93 ± 4.4 mmHg) group and attenuated significantly by the MINO + ASP treatment (90 ± 3.2, p < 0.05, ). Treatment with MINO per se and ASP per se reduced severity of nephropathy but was not significant.
Figure 1. Effects of 4-week treatment with MINO, ASP, and MINO in combination with ASP on creatinine clearance. The values are given as mean ± SD. *p < 0.05, **p < 0.01 when compared with vehicle treated control group.
Renal Histology
Histological slides of kidneys from the diabetic group showed markedly severe destruction in glomerular and tubulointerstitial lesions such as glomerular sclerosis, atrophy, interstitial expansion, and interstitial cellular infiltration as compared with those of the control group. Also, collagen deposition was prominent and tubular spaces were obstructed. In the diabetic rats with the MINO + ASP, general morphology of glomerulus and tubulointerstitial lesions was much improved and showed quite normal appearance.
Tubulointerstitial Fibrosis Index
The kidneys of normoglycemic rats exhibited normal cortical and medullary morphology. Mild tubulointerstitial fibrosis, characterized by accumulation of ECM, tubular dilatation, and atrophy, was observed in the vehicle treated diabetic animals. The tubulointerstitial fibrosis index was 1.6 ± 0.24 in renal cortex and medulla of vehicle treated diabetic rats (). Treatment with MINO plus ASP significantly reduced tubulointerstitial fibrosis (0.6 ± 0.25, p < 0.10) compared to vehicle treated diabetic control.
Table 2. Effects of 4-week treatment with MINO, ASP, and MINO in combination with ASP on renal artery collagen level and renal tubulointerstitial fibrosis index
Histology of Renal Artery
Diabetes causes increase in collagen level in arteries. Diabetes increased collagen level significantly (37.4% ± 2.73) when compared with normoglycemic group (26.9% ± 1.19, p < 0.05). Treatment with MINO per se and ASP per se reduced collagen level slightly but not significantly. Four-week treatment with MINO in combination with ASP significantly decreased (30.8 ± 3.21, p > 0.01) collagen level when compared with diabetic group (37.4 ± 2.73, ).
SDS-PAGE Zymography
Zymogram of kidney homogenate showed high level of 72 kD protein and 92 kD protein in vehicle treated diabetic animals compared to normal animals ( and ). Treatment with MINO per se, ASP per se, and MINO plus ASP reduced the increased level of 72 kD and 92 kD protein. Reduction in 72 kD and 92 kD protein level is more with MINO plus ASP treated group compared to MINO per se and ASP per se treated group ( and ).
DISCUSSION
Diabetic nephropathy is the most common single cause of renal insufficiency. Glomerular changes, such as capillary basement membrane thickening, mesangial proliferation, and nodular glomerulosclerosis, are pathognomic for diabetic nephropathy.Citation17 Tubulointerstitial fibrosis is a predictor of progressive renal failure.Citation18 MMPs have been proposed as important factors driving fibrosis in the glomerulus in various renal diseases, including diabetic nephropathy.Citation19 MMP-2 and MMP-9 belong to the type IV collagenase family of MMPs and are responsible for the degradation of collagen type IV, V, VII, elastin, and fibronectin. Collagen-IV has been identified as the major collagenous component of glomerular basement membrane and ECM.Citation20 As the pathophysiologic actions of MMPs are quite complex and the mechanisms of remodeling appear to be heterogeneous, an abnormal degradation and deposition of ECM may play an important role in the development of structural alterations in glomeruli that contribute to proteinuria.
It is conceivable that abnormalities in collagen-IV degradative pathways take part in the progression of diabetic nephropathy. A role for MMPs in the alteration of basement membrane permeability has been postulated in patients with membranous GN, which reveals elevated levels of TGF-β and collagenase activity in urine.Citation21 Moreover, collagen-IV and fibronectin, which are the major components of ECM, and MMP and TIMP expressions were all altered in diabetic and other nephropathies.Citation22–24 Expansion of ECM in tubulointerstitium and glomerulus is the typical pathological feature of diabetes.Citation25 MMPs also seem to play a crucial role in epithelium–mesenchyme interactions during renal development and determination of nephron number, which can lead to development of chronic renal disease later in life.Citation26
McMillan et al.Citation27 have shown that increased glomerular epithelial cell MMP-9 expression correlates with the period of proteinuria associated with Heymann nephritis and that a direct link exists between glomerular epithelial cell proteolytic activity and loss of glomerular permselectivity. Similarly, MMPs can increase microvascular endothelial permeability. It is also shown that elevated plasma MMP-9 concentration precedes and may predict the development of microalbuminuria in type II DM.Citation28 Furthermore, treatment of nephrotic animals with a synthetic MMP inhibitor has resulted in a reduction of mesangial cell proliferation, ECM deposition, glomerular hypertrophy, and proteinuria.Citation29 These studies strongly suggest a role played by the MMPs in renal diseases such as diabetic nephropathy.
We hypothesized that inhibition of MMP-2 and MMP-9 can attenuate diabetic nephropathy. In our study, 4-week treatment with ASP per se, MINO per se, and ASP plus MINO did not alter blood sugar level and body weight compared to vehicle treated diabetic group which showed that treatment with combination is attenuating diabetic nephropathy through other than glucose-lowering mechanism. This may be due to altered expression of the MMPs and degradation of ECM proteins in the presence of minocycline which is a potent nonselective inhibitor of MMPs and aspirin. STZ-injected rats demonstrated typical characteristics of DM such as hyperglycemia, polyuria, growth retardation, and an increase in urinary albumin excretion. Eight weeks of diabetes induced significant defect in renal function tests, renal hypertrophy, and renal collagen level. Increased blood urea nitrogen and serum creatinine in diabetic rats indicates progressive renal damage which is taken as an index of altered GFR in diabetic nephropathy.Citation30 Treatment with MINO in combination with ASP attenuated renal function tests and renal hypertrophy.
Previous studies have shown that MMPs are involved in the remodeling of the ECM and increase collagen deposition.Citation31 This leads to renal damage and decline in renal function. In the present study, treatment with combination of minocycline and aspirin reduces collagen deposition. This reduction in collagen deposition was more than per se treated group. This reduced remodeling of ECM leads to normalization of vascular structure and reversal of vascular reactivity. Impaired endothelium-dependent vasodilatation has been demonstrated in various vascular beds in diabetic patients and diabetic animals.Citation32 Improved vascular bed leads to amelioration of renal damage which is supported by the histological findings suggesting marked attenuation in renal structures in MINO plus ASP treated group.
In our study, we used MINO in combination with ASP to reduce increased concentration of MMP-2 and MMP-9 in diabetic rats. MINO is a direct inhibitor of MMP-2 and MMP-9. ASP can inhibit MMP-2 and MMP-9 biosynthesis by inhibiting COX-2 and by inhibiting tPA. In diabetics, hyperglycemia induces fibronectin (Fn) overexpression which degrades into proangiogenic Fn fragments (Fn-f). Fn-f has been shown to stimulate tPA-catalyzed plasminogen activation; the plasmin produced then activates proform of MMP-2 and MMP-9.Citation33 Results of zymography suggest that level of 72 kD protein and 92 kD protein which are molecular weights of MMP-2 and MMP-9 are reduced with treatment of MINO plus ASP. Further, these reductions were higher as compared to per se treated groups. Treatment with MINO in combination with ASP attenuated renal function tests which suggested that enhanced inhibition of MMP-2 and MMP-9 by the combination is responsible for improvement.
Tetracycline-like minocycline and doxycycline have been shown to inhibit MMP activity; doxycycline suppresses the activation of proMMP-2 and inhibits the mononuclear phagocyte expression of MMP-9 mRNA. However, Kaito et al.Citation34 reported that doxycycline does not affect MMP-2 production. Although human trials with tetracycline were reported controversially, minocycline has been shown to inhibit MMP-2 and MMP-9 activity in most cases.Citation13
One of the most important aspects of the MMP inhibitors has been the poor correlation between very promising preclinical studies with these drugs and the complete failure of the same drugs when tested clinically. One possible reason could be the vast difference in tumor biology between mouse models and human cancer. As the majority of patients in all of the MMP inhibitor phase III trials corresponded to unresponsive later stage, it is perhaps not surprising that clinical testing of MMP inhibitors failed to replicate the positive results from preclinical studies. Although MMP inhibitors showed poor clinical results, the results of the study by AhujaCitation14 on an incidental reduction of glomerulonephritis proteinuria by 70% with doxycycline treatment in patients revealed therapeutic benefit.
In conclusion, this study demonstrated in experimental diabetic nephropathy that the renoprotective effects of treatment with combination of MINO and ASP that attenuates the accumulation of collagen may, in part, occur via inhibition of MMP-2 and MMP-9. Our study suggests beneficial effects of treatment with combination of MINO and ASP in experimental diabetic neuropathy in rats and can be a potential approach for the treatment.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Atkins RC. The epidemiology of chronic kidney disease. Kidney Int. 2005;67:S14–S18.
- Leehey DJ. Hemodialysis in the diabetic patient with end-stage renal disease. Ren Fail. 1994;16(5):547–553.
- Hostetter TH. Hypertrophy and hyperfunction of the diabetic kidney. J Clin Invest. 2001;107:161–162.
- Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442.
- Williams ME, Tuttle KR. The next generation of diabetic nephropathy therapies: An update. Adv Chronic Kidney Dis. 2005;12:212–222.
- Mauer SM. Structural-functional correlations of diabetic nephropathy. Kidney Int. 1994;45(2):612–622.
- McLennan SV, Kelly DJ, Cox AJ, et al. Decreased matrix degradation in diabetic nephropathy: Effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45(2):268–275.
- Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998;12:1075–1095.
- Yurchenko PD, Schittney JC. Molecular architecture of the basement membrane. FASEB J. 1990;4:1577–1590.
- Petitclerc E, Boutaud A, Prestayko A, New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275(11): 8051–8061.
- Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154.
- Matresian LM. The matrix-degrading metalloproteinases. Bioassays. 1992;14:455–463.
- Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6): 480–498.
- Ahuja TS. Doxycycline decreases proteinuria in glomerulonephritis. Am J Kidney Dis. 2003;42:376–380.
- Bhatt LK, Veeranjaneyulu A. Minocycline with aspirin: A therapeutic approach in the treatment of diabetic neuropathy. Neurol Sci. 2010 [Epub ahead of print] PMID: 20213226.
- Bhatt LK, Veeranjaneyulu A. Attenuation of diabetic retinopathy by enhanced inhibition of MMP-2 and MMP-9 using aspirin and minocycline in streptozotocin-diabetic rats. Am J Transl Res. 2010;2(2):181–189.
- O'Connor AS, Schelling JR. Diabetes and the kidney. Am J Kidney Dis. 2005;46:766–773.
- Kexin XU, Shukun HOU, Zhijun DU. Prognostic value of matrix metalloproteinase-2 and tissue inhibitor of metall- oproteinase-2 in bladder carcinoma. Chinese Med J. 2002; 115(5):743–745.
- Suzuki D. Metalloproteinases in the pathogenesis of diabetic nephropathy. Nephron. 1998;80(2):125–133.
- Vlassara H, Striker LJ, Teichberg S. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci USA. 1994;91:11704 –11708.
- Senatorski G, Paczek L, Sulowicz W. Urine activity of cathepsin B, collagenase and urine excretion of TGF-beta 1 and fibronectin in membranous glomerulonephritis. Res Exp Med (Berl). 1998;198:199–206.
- Zaoui P, Cantin JF, Alimardani-Bessette M. Role of metalloproteases and inhibitors in the occurrence and progression of diabetic renal lesions. Diabetes Metab. 2000;26(suppl. 4):25–29.
- Fogo AB. Current concepts in glomerulosclerosis. Am J Kidney Dis. 1999;34(5):LIV–LVI.
- Kurogi Y. Mesangial cell proliferation inhibitors for the treatment of proliferative glomerular disease. Med Res Rev. 2003;23:15–31.
- Ziyadeh FN. Renal tubular basement membrane and collagen type IV in diabetes mellitus. Kidney Int. 1993;43: 114–120.
- Brenner BM, Anderson S. The interrelationships among filtration surface area, blood pressure, and chronic renal disease. J Cardiovasc Pharmacol. 1992;19(suppl. 6):S1–S7.
- McMillan JI, Riordan JW, Couser WG. Characterization of aglomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. J Clin Invest. 1996;97: 1094–1101.
- Ebihara I, Nakamura T, Shimada N, Koide H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1998;32:544–550.
- Steinmann-Niggli K, Ziswiler R, Kung M, Marti HP. Inhibition of matrix metalloproteinases attenuates anti-Thy1.1 nephritis. J Am Soc Nephrol. 1998;9:397–407.
- Sugimoto K, Tsuruoko S, Fujimura A. Effect of enalapril on diabetic nephropathy in OLETF rats: The role of an anti-oxidative action in its protective properties. Clin Exp Pharmacol Physiol. 2001;28:829–830.
- Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer. Clin Cancer Res. 2003;9(2):551–554.
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974.
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003; 23:117–145.
- Kaito K, Urayama H, Watanabe G. Doxycycline treatment in a model of early abdominal aortic aneurysm. Surg Today. 2003;33:426–433.