Abstract
Background: Protein malnutrition and lowering serum albumin is frequent in hemodialysis patients. A special amino acid formulation has recently been used with favorable effects in elderly people but no data exist in renal patients. Aim: To assess the effects of this novel amino acid formulation in stable hemodialysis patients with reduced albumin levels. Methods: Thirty stable hemodialysis patients with serum albumin levels <3.5 g/dL, normalized protein nitrogen appearance (nPNA) <1.1 g/kg/d, and body mass index (BMI) >20 kg/m2 were selected: 15 patients were randomized to oral amino acid supplementation (4 g thrice a day) for 3 months and 15 patients comparable for age, gender, and dialysis durations formed the control group. Biochemistry and bioimpedentiometry parameters were measured at baseline and at the end of treatment. Results: No difference was observed between study group and control group at baseline. At the end of the study period, no change occurred in the studied parameters in the control group, whereas increase in serum albumin (3.1 ± 0.3 vs. 3.6 ± 0.2 g/dL, p < 0.001) and in total proteins (5.7 ± 0.4 vs. 6.4 ±0.7 g/dL, p < 0.001) occurred in the study group. Hemoglobin rose from 10.7 ± 0.9 to 11.7 ± 0.8 g/dL (p < 0.05) at the same erythropoiesis-stimulating agent (ESA) dosage. C-Reactive protein (CRP) levels decreased in the study group (8.7 ± 7.3 vs. 3.8 ± 3.1 mg/L, p < 0.01). Increase of body weight and of equilibrated protein catabolic rate (ePCR) was observed in the study group. Conclusions: Oral amino acids supplementation was able to improve albumin and total protein in hypoalbuminemia hemodialysis patients. This effect was associated with reduction of CRP levels that is with lowering of pro-inflammatory status and anemia improvement.
INTRODUCTION
Protein-energy malnutrition is quite common in hemodialysis patients and it is associated with reduced physical function and poor quality of life, and also to increased hospitalization, morbidity, and mortality.Citation1–3 Malnutrition may be caused by defective intake of nutrients, the so-called ‘true malnutrition,’ or by a proinflammatory state leading to abnormal body composition with reduction of somatic and visceral protein pool, as a result of a catabolic condition.Citation4 Actually malnutrition and inflammation often coexist in hemodialysis patients and are associated with accelerated atherosclerosis: this condition is known as malnutrition–inflammation–atherosclerosis (MIA) syndrome,Citation5 and it is associated with increased cardiovascular disease and events and hence with a poor clinical outcome in the hemodialysis population.
Assessment of nutritional status is a major task of nephrology care but, unfortunately, several nutritional markers may be affected by other confounding conditions, first of all inflammation. Nutritional guidelines suggest daily energy intake higher than 30–35 kcal/kg ideal body weight and daily protein intake higher than 1.1–1.2 g/kg ideal body weight,Citation6,Citation7 mostly because of the catabolic effect and of the losses of amino acids, peptides, and proteins related to the hemodialysis procedure. Many studies reported that these recommendations are far from being fully obtained;Citation8–10 moreover, chronic renal failure is associated with loss of appetite and reduced food intake, especially when toxins removal by hemodialysis is inadequate.Citation11
Dialysis adequacy and nutritional counseling are the first attempts to ameliorate nutritional status, and then oral nutritional supplements should be employed including energy and protein sources.Citation12 Actually, use of renal-specific oral supplements by hemodialysis patients with low protein intake may prevent a decline in nutritional status and quality of life without increasing the need of phosphate binders.Citation13 Similarly, a branched chain amino acid supplementations have been able to increase serum albumin and to ameliorate nutritional status in hemodialysis patients.Citation14
A special mixture including all of the essential amino acids (EAs) plus two nonessential amino acids (NEAs) (tyrosine and cystine) in a complex ratio that was designed to match metabolic requirements under high demand has been recently investigated in several studies. This amino acid formulation has been reported to exert beneficial effect in elderly people,Citation15,Citation16 in elderly patients affected by chronic heart failure,Citation17 and in patients with type 2 diabetes.Citation18 Moreover, it is known that malnutrition, insulin resistance, and amino acid metabolism are closely related.Citation19 Unfortunately, no data exist about this amino acid supplementation in patients with chronic kidney disease (CKD), who are often elderly, diabetic, or insulin resistant, and with cardiovascular comorbidities.
Therefore, in this pilot study we aimed to assess the effects of this novel amino acid formulation in a cohort of stable hemodialysis patients with reduced albumin levels at risk of malnutrition.
SUBJECTS AND METHODS
This study included patients with serum albumin levels lower than 3.5 g/dL, normalized protein nitrogen appearance (nPNA) <1.1 g/kg/die, and body mass index (BMI) >20 kg/m2, who were on hemodialysis treatment for 6 months at least. All the patients were in stable clinical conditions and free from acute inflammatory or infective events.
Patients with severe cardiac failure (Stage IV NYHA), or respiratory insufficiency, cancer, cachexia, dementia, psychiatric or neurologic diseases, chronic inflammatory systemic diseases, or patients on steroids and/or immunosuppressive drugs were excluded. Hospitalization, surgery, or parenteral nutrition within the last 3 months were considered as exclusion criteria.
Thirty patients were finally selected: 15 patients (5 male, 10 female, aged 72.7 ± 10.2 years, dialysis duration 42.5 ± 38.1 months) were randomized to oral amino acid supplementation treatment; the remaining 15 patients (5 male, 10 female, aged 75.2 ± 11.2 years, dialysis duration 45.1 ± 36.2 months) formed the control group. Underline renal diseases were hypertensive nephroangiosclerosis in 13 cases; diabetic nephropathy in 6 cases; polycystic kidney disease, gold nephropathy, primary amyloidosis, and vasculitis in 1 case; and unknown in 7 cases.
All but one patient concluded the 3-month study period: a female of the control group dropped out the study few weeks after randomization because of bone fracture, so the results of the control group included data from 14 patients.
The patients assigned to the study group received oral amino acid supplementation: 4 g twice a day as a powder dissolved in 100 mL of tap water. The following are the amino acid formulation (Aminotrophic®; Errekappa Euroterapici s.r.l., Milan, Italy) supplies on a daily basis: l-leucine 2500 mg, l-isoleucine 1250 mg, l-lysine 1300 mg, l-valine 1250 mg, l-threonine 700 mg, l-histidine 300 mg, l-cystine 300 mg, l-phenyl-alanine 200 mg, l-methionine 100 mg, l-tyrosine 60 mg, and l-tryptophan 40 mg.
All the selected patients were on a thrice-weekly hemodialysis schedule for 210–240 minutes with synthetic and highly biocompatible membranes. Each patient maintained his/her own dialysis technique that was equally distributed in the control group and in the study group, as well as the vascular accesses: namely, 8 patients were on hemodiafiltration with polysulfone membranes, 2 patients on acetate free-biofiltration with AN69 membrane, 5 on standard bicarbonate dialysis with polysulfone in the study group, 7 patients on hemodiafiltration with polysulfone membranes, 3 on acetate free-biofiltration with AN69 membrane, and 4 diffusive standard bicarbonate dialysis with polysulfone in the control group. In the study group and in the control group, the prevalence of vascular access was the same: native arterovenous fistula in 9 cases, arterovenous graft in 4 cases, and a permanent central vein catheter in 2 cases.
All the patients maintained their own dietary habits and no other oral supplementation of protein or energy was used. Similarly, erythropoiesis-stimulating agents (ESAs) dosage remained unchanged during the study period, as well as the iron administration.
In both the groups at the beginning and at the end of 3 months of follow-up, dialysis-related and nutritional parameters were analyzed.
Nutritional parameters included biochemistry, bioelectric impedance vector analysis (BIVA), height, and body weight before and after-dialysis. BMI was calculated as follows: postdialysis body weight (kg)/height (m2); a BMI value < 20 kg/m2 is considered a sign of malnutrition, and it was consistently associated with the highest mortality risk for dialysis population.Citation2,Citation3
Predialysis biochemical determinations included serum total proteins, albumin, C-reactive protein (CRP), phosphorus, calcium, complement-3 fraction, hemoglobin, and hematocrit. Serum urea level was determined before and after 30 minutes from the end of dialysis treatment, and they were used for the calculation of equilibrated Kt/V (eKt/V) as a measurement of dialysis adequacy and nPNA as an estimation of dietary protein intake. Equilibrated protein catabolic rate (ePCR, g/kg/die) is derived from eKt/V. Erythropoietin resistance index (ERI) was calculated as erythropoietin dosage per week (IU)/kg body weight (kg)/hemoglobin (g/dL).
BIVA was performed at the end of the hemodialysis session using a Bioelectrical Impedance Analyzer (BIA/STA, Akern, Florence, Italy) with a distal, tetrapolar technique, delivering an excitation current at 50 kHz.Citation20 BIVA parameters were measured in duplicate.
BIVA gives two bioelectric parameters: body resistance (R) and reactance (Xc), and the impedance vector (Z) is a combination of R and Xc across tissues. The arc tangent of Xc/R is called phase angle (PA), which is a derived measure obtained from the relation between the direct measures of R and Xc reflecting hydration status and soft tissue cellular mass. Reduced PA reflects increased extra- to intracellular water ratio as well as a decrease in body cell mass; it is a predictor of survival in a number of diseases and also in the dialysis population, where PA values lower than 4.0° are associated with increased mortality risk.Citation21 Blood samples were drawn in fasting condition, before the beginning of the first dialysis of the week, from arterial line.
All the patients gave their informed consent to the study that was approved by the Ethics Committee of the Public Health Authority of Cagliari. The authors declare their adherence to the Declaration of Helsinki.
Statistical Analysis
A statistical package StatView 5 release 5.0.1 for personal computer was utilized for processing data. Descriptive statistics are given as mean ± SD. Statistical analysis was performed by Student's t-test for unpaired and paired data. Linear correlation analysis was performed by Pearson's test. Differences were considered to be statistically significant when p < 0.05.
RESULTS
No difference existed between study group and control group as far as the baseline parameters were concerned (). At the end of the observation period, no change occurred in the study parameters in the control group (). Instead several important changes occurred in the study group, first of all the increase in serum albumin and total proteins (). Actually, serum albumin and total proteins significantly increased, and at the end of the study they were significantly higher with respect to controls.
Table 1. Studied parameters in control and study groups, at baseline and after 3-month study period
Figure 1. Changes of albumin (bottom) and total proteins (top) serum levels (g/dL) in study group (full square) and in the control group (full triangle), at baseline and at the end of the 3‐month study period. **p < 0.001 vs. baseline, *p < 0.05 vs. control group.
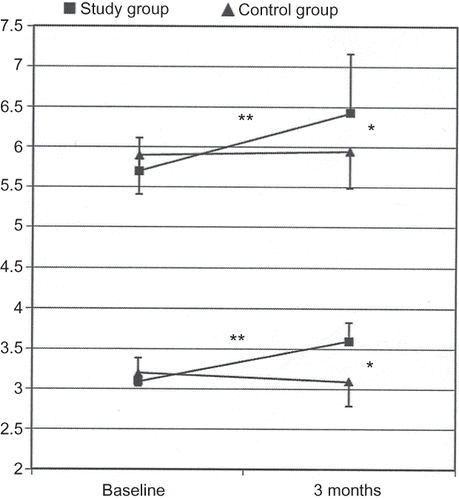
Similarly, hemoglobin increased in the study group and finally reached levels higher than the control group; ERI showed a trend to decrease in study group (). These findings are well in keeping with the decrease of CRP levels (by approximately 47%) we observed in the study group: therefore. amino acid supplemented patients showed lower CRP levels than the control patients at the end of the study ().
No clinical relevant event occurred during the study period. In addition, no side effect was reported in the study group patients: the amino acid supplementation was well accepted by all of them.
No differences were observed between study and control groups in blood urea nitrogen (BUN), plasma levels of sodium, potassium, calcium, phosphorus, glucose, uric acid, as well as total, high- (HDL) and low-density lipoprotein (LDL) cholesterol, leukocytes and lymphocytes, and blood pH and bicarbonate levels. Instead total immunoglobulin significantly increased in the study group but not in the control group; no variation between the two groups in eKt/V but differences were showed in ePCR ().
DISCUSSION
The main finding of this pilot study is the increase in serum albumin and total proteins in a small group of hemodialysis patient induced by oral amino acid supplementation. This occurred together with reduction of indices of inflammation and an improvement of anemia. These changes may be due, in part at least, by amino acid supplementation ‘per se,’ but an increase also in dietary intake cannot be excluded.
The special amino acid formulation we used ontains all the EAs and also two NEAs, namely, tyrosine and cystine, because the former is a NEA only for the liver and the latter prevents methionine from transforming into homocysteine; it represents a source of amino acid nitrogen free from potassium or phosphate.
Albumin synthesis is mostly dependent on sufficient introduction of EAs with food.Citation22 Accordingly, as underlined by Layman, the adequacy of proteins in efficiently supplying nitrogen is related primarily to the amount of EAs provided by them. Therefore, nitrogen supply should not be calculated as the total nitrogen content that is the sum of EAs plus NEAs, but mainly the adequacy of EAs intake. Many pathological conditions increase dramatically nitrogen daily needs, coronary heart disease,Citation23 chronic obstructive pulmonary disease,Citation24 wound healing,Citation25 and traumatized and critically ill patients,Citation26 so that nitrogen intake is impossible to be matched by normal diets.
We believe that performing BIVA at the end of the hemodialysis session is a low-cost, simple, and repeatable tool that is able to give data on body composition and nutritional status. It is noteworthy that changes of PA, free fat mass (FFM), and fat mass (FM) with time are more reliable than cross-sectional data. In the hemodialysis patients, protein requirement is increased. It can be due to amino acids, peptides, and protein losses linked to the hemodialysis technique and procedure. Furthermore, in these groups of patients signs of increased catabolism and inflammation are often present, leading to enhanced nitrogen requirement, and more attention has been given about the changes of ePCR, FM, and FFM. On the other hand, in uremic patients retention of toxins and metabolic derangement may lead to anorexia and so a reduced intake of energy and protein. As a whole, it is not surprising that uremic patients on hemodialysis are at high risk of protein malnutrition. Therefore, supplementation of amino acids may be the first option to counteract loss of lean body mass and visceral proteins.
The mixture of amino acids we used includes all of the EAs plus two NEAs (tyrosine and cystine). It is claimed that it was designed to match metabolic requirements under high demand. It exerted favorable effects on walking capacity, maximal isometric muscular strength, and left ventricular function at rest and during exercise in healthy elderly subjects with reduced physical activity.Citation15
Beneficial results were obtained also in a cohort of elderly patients with chronic heart failure where this oral amino acid supplementation improved exercise capacitiesCitation17 and in patients with type 2 diabetes.Citation18 To our knowledge, no data existed about the use of this amino acid supplementation in patients affected by CKD or in hemodialyzed patients where elderly, insulin resistance, and cardiac disease are quite prevalent. Our preliminary findings suggest that this amino acid formulation may be useful in ameliorating nutritional status in uncomplicated dialysis patients.
The main limitation of the study is the small number of patients: moreover, this is a pilot study that deserves extension with a larger cohort of patients to confirm our stimulating results. Unfortunately, we have no data about the changes in plasma amino acid profiles and we have not been investigated on energy and protein intakes by dietary recalls as well as changes in appetite. Future studies will address these points.
In our opinion, the amino acid formulation has a carefully balanced ratio of EAs for protein synthesis requirements, leading to a rapid improvement in net nitrogen utilization and enhancing visceral proteins synthesis.
CONCLUSIONS
Oral amino acids supplementation was able to improve albumin and total protein in hypoalbuminemia hemodialysis patients. This effect was associated with reduction of CRP levels that is with lowering of proinflammatory status and an improvement of anemia. These stimulating results need confirmation by trials including a larger number of patients.
Acknowledgments
The manuscript has been seen and approved by all authors and that it is not under consideration for publication elsewhere in a similar form, in any language, except in abstract form. No grants and funds were given in support of the study. Ethics committee approval was obtained and the study was in adherence with the Declaration of Helsinki.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
REFERENCES
- Acchiardo SR, Moore LW, Latour PA. Malnutrition as the main factor in morbidity and mortality of hemodialysis patients. Kidney Int. 1983;16:199–203.
- Allen KL, Miskulin D, Yan G, Association of nutritional markers with physical and mental health status in prevalent hemodialysis patients from the HEMO study. J Ren Nutr. 2002;12:160–169.
- Dwyer JT, Larive B, Leung J, Nutritional status affect quality of life in Hemodialysis (HEMO) Study patients at baseline. J Ren Nutr. 2002;12:213–223.
- Mitch WE. Malnutrition: A frequent misdiagnosis for hemodialysis patients. J Clin Invest. 2002;110:437–439.
- Stenvinkel P, Heinburger O, Paultre F, Strong association between malnutrition, inflammation and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000;35(Suppl. 2):S1–S140.
- Fouque D, Vennegoor M, Ter Wee P, EBPG Guidelines on Nutrition. Nephrol Dial Transplant. 2007;22(Suppl.2):S45–S87.
- Bossola M, Muscatiroli M, Tazza L, Variables associated with reduced dietary intake in hemodialysis patients. J Ren Nutr. 2005;15:244–252.
- Cupisti A, D'Alessandro C, Capitanini A, Food intake and nutritional status in stable hemodialysis patients. Ren Fail. 2010;32:47–54.
- Kalantar-Zadeh K, Kopple JD, Deepak S, Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31.
- Burrowes JD, Larive B, Cockram DB, Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: Cross-sectional results from the HEMO study. J Ren Nutr. 2003;13:191–198.
- Bossola M, Muscatiroli M, Tazza L, Malnutrition in hemodialysis patients: What therapy? Am J Kidney Dis. 2005;46:371–386.
- Fouque D, McKenzie J, de Mutsert R, and the Renilon Multicentre Study Group. Use of renal-specific oral supplement by hemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrol Dial Transplant. 2008;23:2902–2910.
- Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic hemodialysis. Nephrol Dial Transplant. 2001;16(9):1856–1862.
- Scognamiglio R, Piccolotto R, Negut C, Tiengo A, Avogaro A. Oral amino acids in elderly subjects: Effect on myocardial function and walking capacity. Gerontology 2005;51:302–308.
- Solerte SB, Gazzaruso C, Bonacasa R, Nutritional supplements with oral amino acid mixture increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E.
- Aquilani R, Viglio S, Iadarola P, Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol. 2008;101:104E–110E.
- Scognamiglio R, Negut C, Palisi M, Dioguardi FS, Coccato M, Iliceto S. Effects of oral amino acid supplements on cardiac function and remodelling in patients with type 2 diabetes with mild-to-moderate left ventricular dysfunction. Am J Cardiol. 2008;101:111E–115E.
- Dioguardi FS. Wasting and the substrate to energy controller pathway: A role for insulin resistance and aminoacids. Am J Cardiol. 2004;93:6A–12A.
- Piccoli A., B. Rossi, L. Pillon A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994;46:534–539.
- Chertow GM, Jacobs DO, Lazarus JM, Lew NL, Lowrie EG, Phase angle predicts survival in hemodialysis patients. J Ren Nutr. 1997;7:204–207.
- Caso M, Feiner J, Mileva I, Response of albumin synthesis to oral nutrients in young and elderly subjects. Am J Clin Nutr. 2007;85:446–451.
- Aquilani R, Opasich C, Gualco A, Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur J Heart Fail. 2008;10(11):1127–1135.
- Koehler F, Doehner W, Hoernig S, Witt C, Anker SD John M. Anorexia in chronic obstructive pulmonary disease – Association to cachexia and hormonal derangement. Int J Cardiol. 2007;119:83–89.
- Demling RH. Nutrition, anabolism, and the wound healing process: An overview. J Plastic Surg Plasty. 2009;9:65–94.
- Soeters PB, van de Poll MCG, Van Gemert WG, Dejong CHC. Amino acid adequacy in pathophysiological states. J Nutr. 2004;134:1575S–1582S.