Abstract
Background: Despite the high prevalence of chronic kidney disease (CKD) in the general population, few CKD patients progress to end-stage renal disease (ESRD). Adding the criterion of proteinuria to the CKD classification could improve screening and therapeutic strategies. Method: We analyzed data from 5122 inpatients who were admitted to our hospital from 2002 to 2003 to survey prevalence of kidney insufficiency, renal survival, mortality, and blood pressure during hospitalization. Results: Among 999 (19.5%) patients with proteinuria of 2+ or more or eGFR under 60 (mL/min/1.73 m2), 56 (9.0%; 95% CI, 6.7–11.4) patients progressed to ESRD (false positive (FP) rate: 18.6%; likelihood ratio (LR): 5.28) and 246 (28.4%; 95% CI, 25.3–31.5) patients died at 5 years. Restricting the focus to patients with proteinuria of 2+ or more or eGFR under 30 reduced the optimal participants by 12.0%, identified 48 (12.4%; 95% CI, 9.0–15.8) patients progressing to ESRD with rising predictive power (FP rate: 11.2%; LR: 7.52) and 162 (29.6%; 95% CI, 25.6–33.5) patients died. The predictors for ESRD were the baseline kidney dysfunction with higher levels of proteinuria, hypertension, and older age. The predictors for death were proteinuria, hypotension, older age, and male. The risk for ESRD differed by levels of proteinuria even though eGFR were in the same levels. In the older CKD inpatients with fewer levels of proteinuria, mortality was raised rather than the rate of the progression to ESRD. Conclusions: Reappraisal by combining proteinuria and eGFR improves prediction of ESRD or death.
INTRODUCTION
In 2002, the National Kidney Foundation, as part of the Kidney Disease Outcomes Quality Initiative (K/DOQI) published clinical practice guidelines on the classification of chronic kidney disease (CKD) based on levels of estimated glomerular filtration rate (eGFR).Citation1 In 2005, Kidney Disease: a position statement on Improving Global Outcomes (K/DIGO) declared that CKD could be classified according to severity, diagnosis, treatment, and prognosis. Eventually, there was agreement with the initial classification as the system was simple and could be linked to ‘action plans’. Meanwhile, it was stated that cause of kidney disease and other risk factors were also important and could be considered in risk stratification.Citation2 Because the cause of kidney disease cannot be ascertained in all cases, the classification system should be useful to most clinicians. Epidemiologically, several studies using this classification system have reported very high prevalence estimates of CKD patients (10.0–13.1%) in the general population.Citation3 In Japan, it was revealed that CKD patients accounted for about 13% (about 13.3 million patients) of the Japanese adult population, which was an unexpectedly large group.Citation4
However, it was reported that few CKD patients progressed to end-stage renal disease (ESRD) despite the high prevalence.Citation5,Citation6 Whether the current CKD criteria and classification are appropriate Citation7–9 and establishing a simple method of ESRD risk assessment that can be applied to all patients with CKD are topics that continue to be discussed.Citation10,Citation11 It is known that the decline of eGFR presents a risk for ESRD, as well as a prelude to hospitalization and death.Citation12 Hospitalization may be a good opportunity to detect the patients suspected of having latent CKD, but the prognosis of these high-risk CKD patients is problematic.
The aims were to determine the prevalence of inpatients with kidney insufficiency and to examine the subsequent prognosis and mortality of these inpatients. In addition, the intent of this study was to discuss whether reappraisal of proteinuria and eGFR at the time of hospitalization is effective in screening for risk of ESRD, and how such screening should be conducted. In addition, we investigated the relationship of the levels of proteinuria and eGFR to blood pressure (BP) during hospitalization.
MATERIALS AND METHODS
Study Population
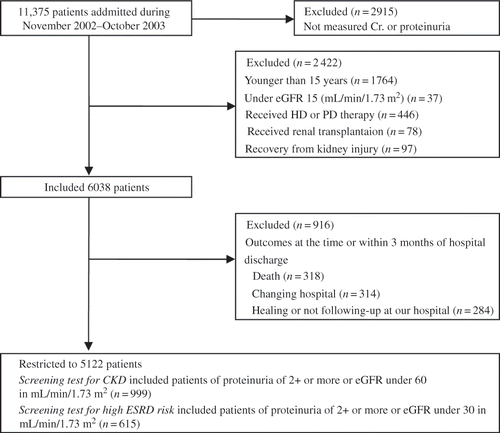
Toho University Omori Medical Center located in the southern part of Tokyo, Japan, is a general hospital with 1021 beds and 27 medical departments comprised of 9 internal medicine, 10 surgical, and 8 other specialities. A total of 11,375 patients were admitted to our hospital in 1 year from 1 November 2002 to 31 October 2003. Study participants were restricted to 5122 patients with the following exclusion criteria: 2915 patients (25.6%) had no dipstick urinalysis test and serum creatinine (Cr.) during hospitalization; 2422 patients were a group that included ‘younger than 15 years’, ‘under eGFR 15 (mL/min/1.73 m2)’, ‘hemodialysis (HD) or peritoneal dialysis (PD) patients’, ‘renal transplantation recipients’, ‘patients recovered from kidney injury at hospital discharge’; and 916 patients for outcomes at the time or within 3 months of hospital discharge, for example, ‘death’, ‘changing hospital’, ‘healing’, or ‘not following-up at our hospital’. The patients who died during hospitalization or within 3 months after hospital discharge were excluded because the kidney damage during hospitalization may have affected mortality. The patients who were not measured for Cr. and proteinuria at 3 months or later after hospital discharge were excluded as ‘not following-up at our hospital’. The patients who recovered completely from kidney injury were excluded as deviations from the aim of this study. Finally, the patients with proteinuria of 2+ or more or eGFR under 60 and the patients with proteinuria of 2+ or more or eGFR under 30 were categorized separately as a screening test for risk of progressing to ESRD. In this study, proteinuria of 2+ or more or eGFR under 30 was assumed as a higher risk indicator of ESRD ().
Study Design
This study is a historical prospective cohort study for the patients who needed hospitalization for some reason. The data for this study were extracted from the patient's electronic files. The study participants were surveyed for age, gender, history of visits to the kidney center, BP during hospitalization, prescription of renin–angiotensin–aldosterone system (RAAS) inhibitors that included angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB), eGFR, and proteinuria by dipstick urinalysis, the eGFR levels were classified according to four stages of eGFR: ≥60, 30–59, 15–29, <15 (mL/min/1.73 m2). Each of these levels was classified by three levels of proteinuria: ± or less, 1+, 2+ or more. The higher value of proteinuria obtained from two or more assays were employed. Each participant was followed up for mortality and annual eGFR until 31 December 2008 (median follow-up, 40.4 ± 24.6 months; range, 3–68 months). ‘Under eGFR 15’ or ‘death’ occurring after discharge was considered as endpoints. Annual eGFR levels were considered as a barometer of kidney dysfunction. The eGFR's calculated using the revision formula of 194 × Cr−1.094 × Age−0.287 (×0.739 for female) for Japanese patients according to the Modification of Diet of Renal Disease (MDRD) methodCitation13 were employed for each average value during hospitalization. The stage based on eGFR levels conformed to the CKD classification proposed by K/DOQI–K/DIGO. The BP employed was the average value obtained during hospitalization. The risk stratification of BP was classified as none (no measurement); low (<100); normal (100–130 and <80); high normal (130–139 or 80–89); Grade 1 (140–159 or 90–99); Grade 2 (160–179 or 100–109); and Grade 3 (≥180 or ≥110) (mmHg). If patients visited the kidney center at least three times (3–38 times) in 2002–2003 and subsequently more than three times (3–28 times) every year, it was designated as ‘referral to nephrologists’.
Ethical Consideration
The ID, names, past history, and information of medical departments were deleted from all subjects and a dataset of observation parameters was extracted only once from electronic media as anonymous information that could not be linked. Maximum attention was paid to respecting the confidentiality of the obtained information and protection from leaking. This study received approval by the institutional ethics committee under requirements for the above (approval number: 18031).
Statistical Analyses
The measured values were expressed as mean ± SD, and the Mann–Whitney's U-test and Fisher's exact test were employed for statistical analysis to compare two independent groups. The Kruskal–Wallis test, followed by Dunn's test, was employed for statistical analysis on more than two independent groups.
Each participant's follow-up time continued until the diagnosis of an endpoint or 31 December 2008. For each endpoint category, the lengths of time to the events were compared by using the log-rank test, and the relative risks of annual transition to downward eGFR levels or death, with 95% confidence intervals (CI), were calculated with the Cox proportional-hazards model. The following multivariate categorical variables were created as the following: age, by every 10-year increment; gender: male or female; every eGFR and proteinuria categorizing decline and excretion; BP: every hypertension categorizing elevation; and prescription of RAAS inhibitors: yes or no. The relative risks were calculated as the hazard ratio (HR) for each reference group in given variables. Age-adjusted associations for available variables were evaluated, and finally, the HRs was adjusted by those multivariate variables. Kaplan–Meier time-to-event methods were employed to estimate the proportion of the each event at 5 years for each category. In addition, a clinical decision analysis was carried out to assess performance as screening for ESRD. The false positive (FP) rate and likelihood ratio (LR) were calculated with sensitivity and specificity (i.e., FP; 1-specificity, LR; sensitivity/1-specificity). A p-value less than 0.05 was considered statistically significant.
RESULTS
Clinical Characteristics of Participants
The relevant baseline characteristics of the 5122 study participants are shown in . The average age of 5122 patients was 56.3 ± 18.4 years, and there were 2753 males and 2369 females. There were 999 (19.5%) patients with proteinuria of 2+ or more or eGFR under 60 whose average age was 65.3 ± 16.1 years. Among these patients, there were 615 (12.0%) patients who were in the higher ESRD risk category with an average age 61.7 ± 17.4 years. Age significantly increased as baseline eGFR declined. Moreover, the distribution of age tended to be older in the eGFR under 60 patients with proteinuria of 1+ or less rather than in those of proteinuria of 2+ or more. No significant difference was noted in gender, but there were many more males than females.
Table 1. Clinical characteristics of 5122 participants classified according to levels of eGFR and proteinuria
The classification of BP levels was distributed among the following: none: 17 (0.3%); low: 149 (2.9%); normal: 2793 (54.5%); high normal: 1183 (23.1%); Grade 1: 849 (16.6%); Grade 2: 120 (2.3%); and Grade 3: 11 (0.2%). The average value of systolic BP (sBP) also increased significantly as baseline eGFR declined. This tendency was more remarkable according to proteinuria levels.
The distribution of prescribed RAAS inhibitors depended on levels of eGFR and proteinuria. The groups receiving prescribed of RAAS inhibitors differed significantly in age (65.0 ± 13.8 vs. 52.8 ± 18.9, p < 0.05) and BP (133 ± 14 vs. 124 ± 13, p < 0.05; 75 ± 9 vs. 73 ± 7, p < 0.05) compared with those groups not receiving the prescribed RAAS inhibitors.
There were 106 (10.6%) patients with proteinuria of 2+ or more or eGFR under 60 who were labeled as ‘referral to nephrologists’. Among these, there were 76 (12.4%) patients with high ESRD risk. In patients of proteinuria of 2+ or more with eGFR over 60, the referral rate was only 8.6%.
Renal Survival Rate and Mortality at 5 Years
The renal survival rate and mortality data derived by Kaplan–Meier time-to-event methods for 5 years are shown in . Overall, 57 (1.7%; 95% CI, 1.3–2.2) patients progressed to eGFR <15, and 671 (16.5%; 95% CI, 15.3–17.6) patients died. Among the 999 (19.5%) patients with proteinuria of 2+ or more or eGFR under 60, 56 (9.0%) patients progressed to eGFR <15 (sensitivity: 98.2%; specificity: 81.4%; FP ratio: 18.6%; LR: 5.28), and 246 (28.4%) patients were deceased at 5 years. Restricting the focus of patients to those with higher risk of ESRD reduced the optimal participants by 615 (12.0%), and identified 48 patients progressing to eGFR <15, and 162 patients who died with rising predictive power for ESRD (sensitivity: 84.2%; specificity: 88.8%; FP ratio: 11.2%; LR: 7.52). However, both screening methods showed high mortality of approximately 30%.
Table 2. Renal survival rate and mortality of participants classified according to levels of eGFR and proteinuria at 5 years
The proportion of progressing to eGFR <15 increased as baseline eGFR declined and was high in patients with proteinuria of 2+ or more. In contrast, the proportion of downward eGFR levels varied depending on proteinuria levels even if baseline kidney function was matched. Mortality increased sharply in the 15–29 eGFR patients with proteinuria of 1+ and 2+ or more. The proportion of the transition to downward eGFR levels exceeded the proportion of ‘death’ in all patients with proteinuria of 2+ or more, in the other two groups of ≥60 eGFR patients, and in the 30–59 eGFR patients with proteinuria of ± or less. The proportion of those progressing to eGFR <15 was higher than the proportion of ‘death’ only in the 15–29 eGFR patients with proteinuria of 2+ or more.
The renal survival rate and mortality on the presence or absence of RAAS inhibitors at 5 years are shown in . The proportion of the transition to downward eGFR levels increased in the groups with RAAS inhibitors than those without in the patients with eGFR greater than 30, or in the 15–29 eGFR patients with proteinuria of 2+ or more. In contrast, the groups with RAAS inhibitors showed decreased mortality in the patients with proteinuria of 2+ or more.
Table 3. Renal survival rate and mortality of participants on prescription of renin–angiotensin–aldsterone system inhibitors at 5 years
Classification of BP on Levels of eGFR and Proteinuria
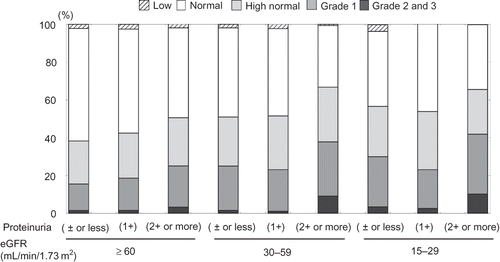
The relationship between classification of average BP during hospitalization and levels of eGFR and proteinuria are shown in . The numbers of patients in proteinuria of 2+ or more or eGFR under 60 and in proteinuria of 2+ or more or eGFR under 30 classified according to BP levels were ‘none: 1 (0.1%)’; ‘low: 18 (1.8%)’; ‘normal: 418 (41.8%)’; ‘high normal: 264 (26.4%)’; ‘Grade 1: 250 (25.0%)’; ‘Grade 2: 44 (4.4%)’ and ‘Grade 3: 4 (0.4%)’ and ‘low: 10 (1.6%)’; ‘normal: 245 (39.8%)’; ‘high normal: 158 (25.7%)’; ‘Grade 1: 161 (26.2%)’; ‘Grade 2: 37 (6.0%)’ and ‘Grade 3: 4 (0.7%)’, respectively. There were the significant differences for the distribution of hypertension in neither. In general, the proportion of patients with more severe hypertension increased in the group with eGFR under 60 or proteinuria of 2+ or more. ‘Age’, ‘gender’, ‘proteinuria by dipstick urinalysis’, and ‘baseline kidney function’ were significant relative risk factors for hypertension. ‘Proteinuria by dipstick urinalysis’ and ‘baseline kidney functions’ were especially significant relative risk factors for hypertension in severe hypertensives.
Figure 2. The relationship between classification of BP and levels of eGFR and proteinuria.
Notes: The risk stratification of BP was classified as ‘low: <100’, ‘normal: 100–130 and <80’, ‘high normal: 130–139 or 80–89’, ‘Grade 1: 140–159 or 90–99’, ‘Grade 2: 160–179 or 100–109’, and ‘Grade 3: ≥180 or ≥110 (mmHg).
The Kidney Risk Factors for Annual Transition to Downward eGFR Levels and Death
The kidney risk factors for annual transition to downward eGFR levels and death are shown in and , respectively. The independent kidney risk factors were ‘≥60; 1+’, ‘≥60; 2+ or more’, ‘30–59; 2+ or more’, ‘15–29; 2+ or more’, ‘Grade 1 hypertension’, and ‘older age’. A few variables (e.g., ‘high normal’ and ‘Grade 2 and 3’ on average BP category) that were the significant risk factors when using univariate analysis turned out to be not significant differences after adjustment by age. Moreover, the kidney risk of ‘30–59; ± or less’ and ‘30–59; 1+’ on univariate changed the inverse association after adjustment by older age or multivariate analysis. The independent risk factors for death were ‘≥60; 1+’, ‘≥60; 2+ or more’, ‘30–59; 1+’, ‘30–59; 2+ or more’, ‘15–29; 1+’, ‘15–29; 2+ or more’, ‘low blood pressure’, ‘older age’, and ‘male’. In contrast, the RAAS inhibitors decreased mortality, and hypertension, rather than normotension, decreased mortality in this study.
Table 4. Multivariate associations between baseline factors and risk for annual transition to downward eGFR levels
Table 5. Multivariate associations between baseline factors and risk for death
DISCUSSION
The current CKD criteria and classification result in a major contribution to the understanding of the comorbidities caused by CKD such as cardiovascular disease (CVD) and mineral and bone disorders (MBD). However, nephrologists have paradox that few CKD patients progressed to ESRD despite the high prevalence.
It is well known that proteinuria is an independent risk factor for ESRD.Citation14–16 However, proteinuria was regarded only as an important related term in the classification of CKD. Therefore, the K/DOQI clinical practice guidelines on CKD have impressed most clinicians rather the importance of eGFR than proteinuria. Iseki et al. reported that the cumulative incidence of ESRD was 1.4% in the screened persons with proteinuria (1+), 7.1% in the screened persons with proteinuria (2+), and 15.4% in the screened persons with proteinuria (3+) for the 17-year follow-up period.Citation14 The distinctive points of our screening for the risk of progressing to ESRD using the combination of proteinuria and eGFR were to detect proteinuria of 2+ or more with eGFR 60 or more and to exclude eGFR 30–59 with proteinuria of 1+ or less. This screening is relatively simple and useful for most clinicians, attracts the attention of other clinicians to proteinuria, can be used efficiently by nephrologists to plan treatment strategies, and can exclude the patients who do not always progress to ESRD. We recommend that the criterion for referral to nephrologists is proteinuria of 2+ or more regardless of eGFR levels, or eGFR under 30.
The combination of eGFR and proteinuria was assessed in some studies.Citation17–19 Iseki et al. studied a total of 95,255 Japanese patients and reported that the cumulative incidence of ESRD was high on a low creatinine clearance (Ccr) with proteinuria by dipstick urinalysis was not as high without proteinuria.Citation17 Hallan et al. reported that their screening by macroalbuminuria in all cases, microalbuminuria with eGFR <60, and normal albumin-to-creatinine ratio (ACR) with eGFR <30 reduced the screening participants from 4.7% to 1.4% compared with those based on CKD stages 3–4 without losing predictive power (69.4% vs. 65.6% of all individuals progressing to ESRD).Citation18 These studies, as well as ours, showed the importance of combining proteinuria and eGFR as the predictive factor for ESRD. Moreover, the Chronic Kidney Disease Prognosis Consortium reported that or GFR less than 60 and ACR 1.1 mg/mmol (10 mg/g) or more are independent predictors of mortality risk in the general population.Citation19 However, our study is different from previous studies in terms of restricting the participants to high-risk inpatients and in terms of concurrently demonstrating about the mortality and the rate of the progression to ESRD. Our screening for the risk of progressing to ERSD at hospitalization demonstrated improvements over previous studies in detection rate, FP rate, and LR than in these studies.
According to our results, there were the anomalous results in which the HR for the decline of eGFR on ‘30–59; ± or less’ and ‘30–59; 1+’ shown an inverse association after adjustment by older age or multivariate analysis. The reasons considered for this were the kidney function during or after hospitalization might fluctuate because of underlying diseases or various complications; the inpatients showing decline of eGFR without higher levels of proteinuria were obviously of older age in comparison with the inpatients without decline of eGFR; and the baseline kidney dysfunction of proteinuria of 1+ or less was a rather risk factor for death than the risk of progressing to ESRD. Imai et al. reported the individuals of eGFR <40 with age 70–79 were the risk of progressing to ESRD in the Japanese general population.Citation20 However, this study did not discuss about levels of proteinuria.
The Grade 1 hypertension was a kidney risk factor, but the Grade 2 and 3 hypertensions were not significant kidney risk factors. Hypotension was related with mortality, while hypertension was associated with lower mortality rather than normotension. Jafar et al. reported that hypertension and hypotension with high levels of proteinuria were higher risks for kidney function progression.Citation21 This discrepancy may have occurred because BP in our study was affected by unstable conditions in hospitalization, or because the normotension caused by a drop in BP associated with these conditions were not excluded. In fact, the diseases triggered hypertension during the acute phase (e.g., stroke, subarachnoid hemorrhage, pregnancy-induced hypertension, preeclampsia, hypertensive emergency, and hypertensive urgency) were included 15.3% in the patients with Grade 2 and 3 hypertensions. The bleeding and the dehydration bringing about a drop in BP were found in the patients with normotension. The contradiction of the effect of RAAS inhibitors for kidney function may be explained by a mismatch of patients on the use (or non-use) of the prescribed drug (e.g., age, baseline BP, and underlying disease). Casas et al. reported that additional renoprotective actions of RAAS inhibitors beyond lowering blood pressure remain unproven in patients with diabetes, and that there is uncertainty about the greater renoprotection seen in non-diabetic renal disease.Citation22 Our results suggest that the renoprotection of RAAS inhibitors remain unproven beyond influences of age, baseline BP, and underlying disease, even if levels of baseline eGFR and proteinuria are matched. On the other hand, the RAAS inhibitors improved mortality in patients with risk of kidney dysfunction. This result suggests the possibility that the RAAS inhibitors worked protectively for cardiovascular problems in hospitalized patients.
The following problems have been raised in our study. (a) Although Cr. and proteinuria during hospitalization were extracted, all patients did not completely satisfy the criteria of CKD. (b) The levels of proteinuria were classified using dipstick urinalysis. The proteinuria in hospitalized patients may be affected by various medical comorbidities and side effects of many medications. Therefore, it remains possible that false positive or negative results were included in the dipstick urinalysis for proteinuria. However, the quantitative value of proteinuria or microalbuminuria might be unsuitable for screening because except for nephrologists, most clinicians, rarely measure the quantitative value of proteinuria. Also, the higher levels of proteinuria were associated with subsequent renal survival and mortality in this study. (c) As BP during hospitalization was extracted, there was a possibility that BP was a little higher due to stress of hospitalization. (d) This study did not identify the cause of kidney disease. The K/DOQI clinical practice guidelines on CKD provided the definition of CKD independent of cause. It is clear that the underlying disease cannot be ascertained in all cases. One of the aims in this study was to devise a strategy to care for inpatients with kidney insufficiency. (e) This study did not identify the cause of death, nor was it the principal aim of this study. (f) Since this was a tertiary care university hospital study, the results could be different among other hospitals such as municipal, private, or public hospitals, and the procedures of clinical practice described here may not be appropriate for nationwide use.
We conclude that the detection of the high ESRD risk inpatients with the criterion of proteinuria of 2+ or more regardless of eGFR levels or with eGFR under 30 could and more efficiently link to ‘action plans’ for prevention against ESRD. In the older eGFR under 60 inpatients with proteinuria of 1+ or less, mortality was raised rather than the rate of the progression to ESRD. Reappraisal of proteinuria and eGFR improves prediction of ESRD or death in hospitalized patients.
Acknowledgements
We wish to express our deep appreciation to Ryoichi Asayama in the Division of Electronic Calculation and Toshihiko Ota in the Division of medical record administration, Purser Department in our center, for cooperation to extract electronic information.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis Suppl. 2002;39:S1–S266.
- Levey AS, Eckardt KU, Tsukamoto Y, Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100.
- Coresh J, Selvin E, Stevens LA, Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007;298:2038–2047.
- Imai E, Horio M, Watanabe T, Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–630.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663.
- Hallan SI, Coresh J, Astor BC, International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–2284.
- Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: Time for a course correction. J Am Soc Nephrol. 2008;19:844–866.
- Couser WG. Chronic kidney disease the promise and the perils. J Am Soc Nephrol. 2007;18:2803–2805.
- de Jong PE, Gansevoort RT. Fact or fiction of the epidemic of chronic kidney disease – let us not squabble about estimated GFR only, but also focus on albuminuria. Nephrol Dial Transplant. 2008;23:1092–1095.
- Crowe E, Halpin D, Stevens P. Early identification and management of chronic kidney disease: Summary of NICE guidance. BMJ. 2008;337:a1530.
- Taal MW, Brenner BM. Renal risk scores: Progress and prospects. Kidney Int. 2008;73:1216–1219.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305.
- Matsuo S, Imai E, Horio Y, Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992.
- Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–1474.
- Ishani A, Grandits GA, Grimm RH. Association of single measurements of dipstick proteinuria estimated glomerular filtration rate and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452.
- Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE, PREVEND Study Group. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl. 2004;92:S18–S21.
- Iseki K, Kinjo K, Iseki C,, Takishita S. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis. 2004;44:806–814.
- Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR, Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077.
- Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all‐ cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–2081.
- Imai E, Horio M, Yamagata K, Slower decline of glomerular filtration rate in the Japanese general population: A longitudinal 10-year follow-up study. Hypertens Res. 2008;31:433–441.
- Jafar TH, Stark PC, Schmid CH, Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med. 2003;139:244–252.
- Casas JP, Chua W. Loukogeorgakis S, Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: Systematic review and meta-analysis. Lancet. 2005;366:2026–2033.