Abstract
Background: Advanced glycation end product (AGE) levels are elevated in patients with decreased renal function and may contribute to the excessive cardiovascular disease in this population. However, their relation to nutrition, anemia, and micro inflammation is not well characterized. The aim of this study is to determine their relationship in patients with chronic kidney disease (CKD). Methods: The studied group consisted of 203 subjects: 159 patients with CKD 1–5 and 44 healthy control subjects. AGE levels were assessed by spectrofluorimetry, and routine biochemical parameters were measured using standard methods. Results: AGE levels were significantly increased in CKD patients compared with controls (3.9 ± 1.7 × 105 AU in CKD 1–5 patients vs. 3.2 ± 0.48 × 105 AU in controls, p < 0.0001). AGE levels increased from CKD 3. AGE levels were positively associated with age, albumin, prealbumin, and orosomucoid, and were negatively associated with hemoglobin and estimated glomerular filtration rate. In multiple regression analysis, after adjustment to age and glomerular filtration rate, AGE levels remained independently correlated with albumin and prealbumin and negatively correlated with hemoglobin. Conclusions: This study is the demonstration that nutrition markers, albumin and prealbumin, are the positive determinants and hemoglobin is the negative determinant of serum AGE levels in patients with CKD.
INTRODUCTION
Chronic kidney disease (CKD) is associated with enhanced oxidative stress, which is assumed to accelerate the progression of renal and cardiovascular disease.Citation1,Citation2 The factors that affect the progression of CKD have been incompletely characterized. Advanced glycation end products (AGEs), the short- and long-term modification products of glycation or glycoxidation of proteins and lipids,Citation3,Citation4 are implicated in the pathogenesis of CKD, diabetes, and atherosclerosis.Citation5,Citation6
AGE toxicity may occur through at least three mechanisms: interaction with the receptor for AGEs (RAGE), tissue deposition, and in situ glycation. AGEs trigger proinflammatory, profibrotic, and procoagulant cellular responses that are capable of damaging tissues, often targeting particular organs. In diabetic patients, the conditions needed to promote AGE formation are all present and are further accentuated by accompanying renal failure.Citation7
AGEs are also implicated in the pathophysiology of nondiabetic renal diseases and aging. AGEs have been found in renal tissues in IgA nephritis and lupus nephritis, hypertensive nephropathy, renal fibrosis, and chronic transplant rejection.Citation6,Citation8,Citation9 The pivotal role of AGEs during glomerular and tubulointerstitial damage and in the development of complications due to decreased renal function is now increasingly recognized as well as their role in promoting local and inflammatory response in the kidney. The precise role in these different kidney pathologies has yet to be elucidated. In nondiabetic patients, the presence of AGEs could disrupt the balance between dietary intake, oxidation, in situ glycation, and renal elimination.
Oxidative stress, which occurs when there is excessive free-radical production or low antioxidant level, is implicated to be a factor for the development of endothelial dysfunction and atherogenesis.Citation10 In this context, it is of interest that inflammatory response is associated with decreased levels of several antioxidants.Citation11 Similarly, during malnutrition, increased oxidative stress, in combination with chronic inflammation, and anemia might lead to increased risk of cardiovascular and renal disease.
Formation of AGEs is a ubiquitous process of protein modification and degeneration by sugar molecules and reactive intermediates of oxidant and carbonyl stress that occurs in situations where the biochemical equilibrium is shifted toward covalent interaction between the reaction partners. This effect is most prominent in diabetes mellitus (DM) and chronic renal failure characterized by uremic carbonyl stress when considerable amount of AGE-modified proteins accumulate in plasma and tissues.Citation6
Despite advances in renal pathology, many important aspects of AGEs, their role in renal biology, and determinants of their levels still remain unclear.
This study aims to examine correlates of AGEs measured in a cohort of patients with CKD stages 1–5, not yet requiring dialysis. Specifically, we examine for the relationships between AGEs and markers of nutrition, anemia, and micro inflammation, whose associations have been previously reported or are biologically plausible.
SUBJECTS AND METHODS
Subjects
We recruited 159 patients at different stages of CKD at the Department of Nephrology (Charles University and General Faculty Hospital, Prague, Czech Republic). The control group consisted of 44 healthy subjects. Written informed consent and laboratory samples were obtained from all subjects according to ethical guidelines. This study was approved by the local Institutional Ethical Committee.
Clinical, demographic, and biochemical characteristics of the group are presented in . The etiology of CKD was the following: Anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (20%), hypertensive nephropathy (8%), lupus nephritis (14%), IgA nephritis (21%), diabetic nephropathy (4%), membranous nephropathy (15%), focal and segmental glomerulosclerosis or minimal change disease (11%), amyloidosis (5%), and other (1%). Patients were separated into five CKD groups according to their estimated glomerular filtration rate [eGFR; Modification of Diet in Renal Disease (MDRD)], based on Kidney Dialysis Outcomes Quality Initiative (K/DOQI), as follows: stage 5 (eGFR < 15 mL/min), stage 4 (eGFR 15–30 mL/min), stage 3 (30–60 mL/min); patients with CKD stage 1 and 2 were analyzed together (eGFR > 60 mL/min). History of cardiovascular disease (CVD) was taken from medical records of each patient, comprising ischemic heart disease, peripheral ischemic disease, and/or cerebrovascular disease. Most patients received medications commonly used in patients with CKD, such as diuretics, antiplatelet drugs, calcium and vitamin D supplements, statins, and antihypertensive drugs.
Table 1. Clinical, demographic, and laboratory characteristics of the study group and controls
Methods
Blood samples
Fasting blood samples from each patient were collected through puncture of the cubital vein simultaneously with blood collection for routine examination. All blood samples were centrifuged for 10 min at 1.450 g (4°C). Sera were stored at −80°C until analysis.
Biochemical analysis
Determination of fluorescent AGE is based on spectrofluorometric detection according to Henle et al.Citation12 and Munch et al.Citation13 Blood serum was diluted 1:50 with phosphate-buffered saline, pH 7.4, and fluorescence intensity was recorded at the emission maximum (≈440 nm) upon excitation at 350 nm (Fluoromax-3 spectrofluorometer, Jobin Yvon Horiba, Edison, New Jersey, USA). Fluorescence intensity was expressed in arbitrary units as AU.
C-reactive protein (CRP) was determined turbidimetrically, orosomucoid (acidic α1-glycoprotein) was assessed nephelometrically, and fibrinogen was measured by the thrombin method (Clauss).
Routine biochemical parameters were assessed using standard laboratory methods. Blood count was measured with an automated hematological analyzer. The eGFR was calculated using the MDRD formula.Citation14
Statistics
Statistical analyses were performed using Statistics Toolbox™ MATLAB® software (The MathWorks™, Inc., Natick, Massachusetts, USA). Data are presented as the mean ± SD for continuous variables and percentages for categorical variables. Comparisons between groups were conducted with unpaired sample t-tests and analysis of variance (ANOVA) for normally distributed continuous variables and Mann–Whitney U test and Kruskal–Wallis ANOVA for non-normal distributions. Variables with non-normal distributions were log-transformed where appropriate. Association among analyzed parameters was assessed using Spearman's or Pearson's correlation coefficient. All results were considered statistically significant at p < 0.05.
RESULTS
illustrates the clinical, demographic, and laboratory data of the study group and controls. There was a trend to higher BMI for CKD 4 and CKD 5 compared with the controls (p = 0.02).
Mean serum AGE concentrations at different stages of CKD were 3.9 ± 1.7 × 105 AU. Serum AGE levels were increased from CKD 3 (3.7 ± 1.4 × 105 AU, p < 0.0001) as well as there was an increase in orosomucoid levels (1.25 ± 0.56 g/L, p < 0.0001).
Moreover, patients with different stages of CKD with CVD, compared with those patients without CVD, had comparable AGE levels. Similarly, in patients with different stages of CKD with DM, there was no difference of AGE levels compared with those in patients without DM.
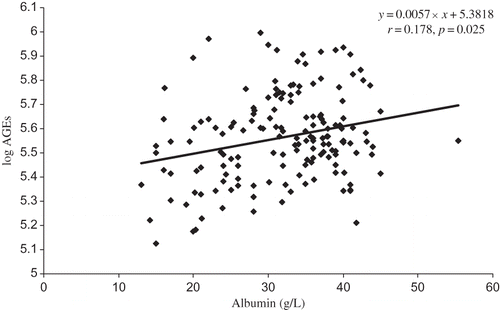
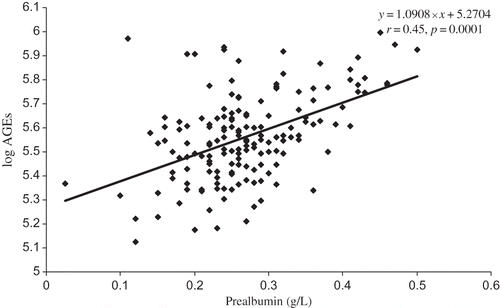
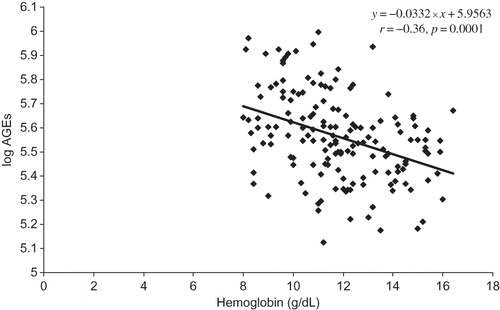
Parameters statistically and significantly related to AGE levels were age (r = 0.206, p = 0.009), albumin (r = 0.178, p = 0.025) (), prealbumin (r = 0.45, p = 0.0001) (), and orosomucoid (r = 0.269, p = 0.001) and those inversely related are eGFR (r = −0.517, p = 0.0001) and hemoglobin (r = −0.36, p = 0.0001) ().
Multiple regression analysis was applied to evaluate the major factors that affect AGE levels in this study population. Finally, albumin (p = 0.0006), prealbumin (p < 0.0001), and hemoglobin (inversely p < 0.0001) even after adjustment for age and eGFR remained significant () and were independently related to AGE levels (R2 = 0.45).
Table 2. Factors associated with AGEs. Multivariate regression analysis using serum AGEs as the dependent variable (adjusted to age and eGFR)
Taken together, increased levels of AGEs are associated with nutrition markers, albumin and prealbumin, and lower hemoglobin in CKD 1–5 patients of various nephropathies not yet undergoing dialysis.
DISCUSSION
The most important finding in this study is the demonstration of the link between nutrition markers, anemia, and fluorescent AGE levels in CKD 1–5 patients of various nephropathies not yet undergoing dialysis. AGE levels were positively associated with albumin and prealbumin and negatively with hemoglobin, important risk markers for malnutrition, inflammation, atherosclerosis, and anemia.Citation2,Citation15–17
We focused our analysis to subjects with various nephropathies not yet dialyzed for the following reasons. First, renal disorders linked with AGEs are reported not only during diabetic nephropathy but also during chronic renal failure, chronic inflammatory disease, high dietary AGE, and aging.Citation18–23 Second, among 159 subjects enrolled in this study, only 14% patients had diabetes. Third, there is a possibility of association between AGE levels, markers of nutrition, and anemia if they are analyzed all together. Finally, AGEs are known to be important in atherosclerosis progression and are positively correlated with cardiovascular mortality in nondiabetic women.Citation24
There have been several studies reporting serum AGE levels in uremic patients. Some authors report no difference in the serum AGE levels in the patients with and without DM on chronic dialysis treatment.Citation13,Citation25 On the contrary, other authorsCitation26–28 described higher AGE levels in dialyzed patients with DM. Additionally, AGE levels in uremic patients were found to be several times higher than in patients with diabetes and normal kidney function. Mechanisms other than hyperglycemia therefore must be involved.Citation29 In this study, the AGE levels in CKD patients with DM and in those patients without DM were not different. The interpretation is difficult because the studied group of patients with DM was not large. Nevertheless, this finding suggests that the decreased AGE disposal in CKD patients is more essential for accumulation of AGEs than long-term glycemia in those CKD patients with DM.
In our study, AGE levels were inversely related to eGFR. The increase in AGE levels was evident from CKD 3. In CKD patients, the reason for the increase in AGEs is a decreased ability of the kidney to clear AGEs from circulationCitation23 and also increased carbonyl stress, a major pathogenic mechanism of AGE formation and risk factor for patients with CKD.Citation30
In hemodialysis patients, there is a controversy whether high AGE levels or other confounders including better nutritional status and a lower incidence of preexisting vascular disease influence better survival of hemodialysis patients.Citation31,Citation32 Our study showed no difference in AGE levels of CKD patients not yet dialyzed with CVD and those without CVD, but a relationship to nutritional status as in hemodialysis patients was found also in CKD patients not yet dialyzed. Similarly as in hemodialysis patients, it needs to be elucidated whether the benefit of high-serum AGEs is an epiphenomenon or reflects a better nutritional status also in CKD patients.
BMIs for CKD 4 and CKD 5 were significantly higher, implying that the subjects in these groups were overweight. Obesity is a known high-risk factor for the development of vascular diseases and CKD. Also, increased expression of proinflammatory cytokines and increased infiltration of immunocompetent cells were found in the obese patients with CKD stages 3–4.Citation33 It remains to be determined how important a rising BMI is in the association of AGEs with nutrition markers in the progressive stages of CKD.
Very limited data are present in patients with CKD not yet undergoing renal replacement therapy. In this study, in patients with CKD not yet dialyzed, we found a positive relationship of AGEs and age, albumin, prealbumin, and orosomucoid, whereas a negative relationship between AGEs and eGFR and hemoglobin was noted. This finding implies that a complex interplay between renal function, nutrition parameters, inflammation, and AGE levels exists in patients with various types of nephropathy. Indeed, circulating AGEs are linked to plasma proteins, mainly albumin,Citation29 and AGE accumulation in plasma proteins could be attributed to a gradual decrease in renal clearance of protein-linked AGEs. In this study, serum AGE levels were not correlated with proteinuria, but the increased levels of AGEs might implicate detrimental effects on the kidney inducing an increase in proteinuria in patients with CKD.
Individuals with CKD become progressively malnourished, as evidenced by low levels of albumin, prealbumin, and transferrin, which has been suggested to be a mechanism for activation of inflammation.Citation34 Malnutrition and chronic inflammation often coexist in CKD patients not yet on maintenance dialysis.Citation34 CRP and orosomucoid were shown to be the inflammatory markers in different stages of CKD.Citation35 AGEs might describe long-lasting oxidative damage.Citation36 Although there was no relation between AGE levels and CRP in a cohort of our patients, probably due to the high interindividual variability, a relationship between AGE levels and orosomucoid was found. A simultaneous increase of AGE levels and orosomucoid with decrease of renal function was shown. Thus the impact of micro inflammation and oxidative stress on AGE levels cannot be excluded in CKD patients not yet dialyzed.
In accordance with the study of Thomas et al.,Citation37 we also found a close association between declining hemoglobin and AGEs. It is not clear how hemoglobin levels influence either the production or the clearance of AGEs, but both hemoglobin and AGEs are markers of kidney injury. Certainly, anemia in CKD identifies patients at increased risk for progressive renal disease, hospitalization, and premature death.Citation38,Citation39 Reduced tissue oxygenation associated with anemia may contribute to the formation of AGEs. Given the continuous relationship between hemoglobin and renal function, this association deserves further studies.
In our multiple regression model, albumin, prealbumin, and negatively hemoglobin, after the adjustment for age and eGFR, are independent determinants of fluorescent AGEs, supporting the notion that both low hemoglobin and nutrition parameters determine their levels in CKD patients. It is conceivable that AGEs might contribute to some of the risk for nutrition abnormalities and anemia associated with renal impairment.
Our study was limited by its observational nature, which allowed us to establish associations, but not causality. This study involved patients with various nephropathies with comorbidities, and the findings cannot necessarily be extrapolated to less morbid subjects. Dietary intake of AGEs was not assessed in this study. Angiotensin-converting enzyme-1 inhibitors are also another factor that may potentially modulate the AGE–RAGE pathway.Citation40
In conclusion, increased levels of AGEs are associated with nutrition markers, albumin and prealbumin, and lower hemoglobin and appear, at least, to partly modulate the development of malnutrition and anemia in CKD patients. Additional studies are needed to determine whether increased AGEs predict a development of malnutrition, inflammation, and atherosclerosis syndrome in patients with CKD.
Acknowledgments
This study was supported by research project MSM 0021620807. The authors are thankful to the laboratory staff for their technical assistance.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Siems W, Quast S, Carluccio F, Oxidative stress in chronic renal failure as a cardiovascular risk factor. Clin Nephrol. 2002;58(Suppl. 1):S12–S19.
- Stenvinkel P, Heimbürger O, Paultre F, Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911.
- Brownlee M. Advanced glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234.
- Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon- (carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271: 9982–9986.
- Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592.
- Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289:F645–F659.
- Daroux M, Prévost G, Maillard-Lefebvre H, Advanced glycation end-products: Implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 2010;36:1–10.
- Hartog JW, Smit AJ, van Son WJ, Advanced glycation end products in kidney transplant patients: A putative role in the development of chronic renal transplant dysfunction. Am J Kidney Dis. 2004;43:966–975.
- Berrou J, Tostivint I, Verrecchia F, Advanced glycation end products regulate extracellular matrix protein and protease expression by human glomerular mesangial cells. Int J Mol Med. 2009;23:513–520.
- Halliwell B. The role of oxygen radicals in human disease with particular reference to vascular system. Hemostasis. 1993;23 (Suppl. 1):118–126.
- Boosalis MG, Snowdon DA, Tully CL, Gross MD. Acute phase response and plasma carotenoid concentrations in older women: Findings from the nun study. Nutrition. 1996 Jul–Aug; 12(7–8):475–478.
- Henle T, Deppisch R, Beck W, Hergesell O, Hänsch GM, Ritz E. Advanced glycated end-products (AGE) during hemodialysis treatment: Discrepant results with different methodologies reflecting the heterogeneity of AGE compounds. Nephrol Dial Transplant. 1999;14:1968–1975.
- Münch G, Keis R, Wessels A, Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur J Clin Chem Clin Biochem. 1997;35:669–677.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470.
- Riella MC. Malnutrition in dialysis: Malnourishment or uremic inflammatory response? Kidney Int. 2000;57:1211–1232.
- Caravaca F, Arrobas M, Pizarro JL, Sanchez-Casado E. Uraemic symptoms, nutritional status and renal function in pre-dialysis end-stage renal failure patients. Nephrol Dial Transplant. 2001;16:776–782.
- Jansen MA, Korevaar JC, Dekker FW, Renal function and nutritional status at the start of chronic dialysis treatment. J Am Soc Nephrol. 2001;12:157–163.
- Wautier JL, Schmidt AM. Protein glycation: A firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238.
- Horie K, Miyata T, Maeda K, Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997;100:2995–3004.
- Yamada K, Miyahara Y, Hamaguchi K, Immunohistochemical study of human advanced glycosylation end-products (AGE) in chronic renal failure. Immunohistochemical study of human advanced glycosylation end-products (AGE) in chronic renal failure. Clin Nephrol. 1994;42:354–361.
- Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A. 1994;91:11704–11708.
- Gugliucci A, Bendayan M. Reaction of advanced glycation endproducts with renal tissue from normal and streptozotocin-induced diabetic rats: An ultrastructural study using colloidal gold cytochemistry. J Histochem Cytochem. 1995;43: 591–600.
- Semba RD, Ferrucci L, Fink JC, Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis. 2009;53:51–58.
- Kilhovd BK, Juutilainen A, Lehto S, High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: A population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol. 2005;25:815–820.
- Papanastasiou P, Grass L, Rodela H, Patrikarea A, Oreopoulos D, Diamandis EP. Immunological quantification of advanced glycosylation end-products in the serum of patients on hemodialysis or CAPD. Kidney Int. 1994;46:216–222.
- Makita Z, Bucala R, Rayfield EJ, Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994;343:1519–1522.
- Vlassara H. Serum advanced glycosylation end products: A new class of uremic toxins? Blood Purif. 1994;12:54–59.
- Kalousová M, Zima T, Tesař V, Glycoxidation in hemodialyzed patients with diabetes mellitus. In: Timio M, Wiyemann V, Venanzi, eds. Cardionephrology. Vol. 7. Cosenza, Italy: Editoriale Bios; 2002:297–300.
- Miyata T, Fu MX, Kurokawa K, van Ypersele de Strihou C, Thorpe SR, Baynes JW. Autoxidation products of both carbohydrates and lipids are increased in uremic plasma: Is there oxidative stress in uremia? Kidney Int. 1998;54: 1290–1295.
- Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999;55:389–399.
- Schwedler SB, Metzger T, Schinzel R, Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002;62:301–310.
- Wagner Z, Molnár M, Molnár GA, Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis. 2006;47:294–300.
- Teplan V, Vyhnálek F, Gürlich R, Increased proinflammatory cytokine production in adipose tissue of obese patients with chronic kidney disease. Wien Klin Wochenschr. 2010; 122(15–16):466–473.
- Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000;15:953–960.
- Romão JE Jr, Haiashi AR, Elias RM, Positive acute-phase inflammatory markers in different stages of chronic kidney disease. Am J Nephrol. 2006;26:59–66.
- Kalousová M, Sulková S, Fialová L, Glycoxidation and inflammation in chronic hemodialysis patients. Nephrol Dial Transplant. 2003;18:2577–2581.
- Thomas MC, Tsalamandris C, MacIsaac R, Low-molecular-weight AGEs are associated with GFR and anemia in patients with type 2 diabetes. Kidney Int. 2004;66:1167–1172.
- Holland DC, Lam M. Predictors of hospitalization and death among pre-dialysis patients: A retrospective cohort study. Nephrol Dial Transplant. 2000;15:650–658.
- McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: A population-based study. J Am Soc Nephrol. 2002;13:1928–1936.
- Forbes JM, Thorpe SR, Thallas-Bonke V, Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16:2363–2372.