Abstract
Background: There are limited studies describing various renal disorders and their prognostic impact in patients with cirrhosis of liver. The aim of this work was to study the clinical spectrum of renal disorders in patients with cirrhosis of liver and their prognostic impact. Methods: Patients with diagnosis of cirrhosis of liver were included in this study. Cirrhosis was diagnosed using standard clinical criteria. The cirrhotic patients were prospectively analyzed for the presence of renal diseases during the study period from January 2008 to April 2009. Results: Four hundred and four patients were included in this study and renal diseases were present in 44% (n = 178) patients. The spectrum of renal diseases were acute kidney injury (AKI; 24.5%), chronic kidney disease (CKD; 15.6%), acute on chronic renal failure (1.5%), nephritic syndrome (1.5%), and nephrotic syndrome (1%). The types of AKI were acute tubular necrosis (ATN; 44.4%), prerenal failure (36.4%), and hepatorenal syndrome (19.2%). The incidence of renal diseases was 15.7% in class A, 50% in class B, and 54.8% in class C cirrhosis. There was significant increase in mortality in patients with class C cirrhosis versus without renal disease (78.1% vs. 53.2%; p < 0.001). Conclusions: Renal diseases were present in a significant proportion (44%) of cirrhotic patients. ATN was the commonest form of AKI and we noted that the prevalence of CKD was 15.6% in our cirrhotic patients. The incidence of renal disease increased with increase in severity of cirrhosis of liver. The presence of renal disease seems to have adverse prognostic impact on class C cirrhosis.
INTRODUCTION
The clinical course of patients with cirrhosis is often complicated by a number of important sequelae that can occur regardless of the underlying cause of the liver disease.Citation1 Renal disorder is a common and serious problem in patients with cirrhosis of liver. The exact incidence of renal disorders in patients with cirrhosis is largely unknown and probably underestimated,Citation2 whereas the prevalence of chronic kidney disease (CKD) in patients with cirrhosis has not been reported.Citation3 Most of these studiesCitation4,Citation5 are retrospective and/or they address only a particular subset of renal disorders [e.g., acute renal failure and hepatorenal syndrome (HRS)] rather than the whole spectrum of renal diseases that occurs in cirrhotic patients. This prospective and hospital-based study was undertaken to address the incidence, etiology, and spectrum of renal disorders in patients with cirrhosis of liver and their impact on prognosis.
SUBJECTS AND METHODS
This study was carried at Sir Sunder Lal Hospital, Institute of Medical Sciences, Banaras Hindu University, a tertiary referral center in Varanasi, India, from January 2008 to April 2009. The patients with clinical diagnosis of cirrhosis of liver were evaluated for the presence of clinical renal diseases. Cirrhosis of liver was diagnosed on the basis of the presence of both portal hypertension and hepatocellular dysfunction and supplemented by imaging studies.Citation6 The diagnosis of cirrhosis of liver was considered positive only when no possible explanation for portal hypertension other than cirrhosis was present.
The following data were collected: age, sex, presenting complaint, viral markers, and etiology of cirrhosis, complete blood count, renal and liver function tests, ascitic fluid analysis, and coagulation profile. Radiological imaging and other tests (e.g., upper GI endoscopy, serum ceruloplasmin) required for diagnosis and management of the patients were carried in selected cases. Staging was done using modified Child–Pugh scoring system.Citation7 All patients were evaluated for clinical evidences of renal disorder. The renal diseases were grouped as (1) acute renal failure; (2) CKD; (3) acute on chronic renal failure; (4) nephrotic syndrome, and (5) nephritic syndrome. The patients with acute renal failure were further divided as prerenal failure, HRS, and acute tubular necrosis (ATN).
Definitions used for the purpose of this study were:
Acute renal failure: Reduction in kidney function manifested by an absolute increase in serum creatinine of 0.3 mg/dL or more, equivalent to a percentage increase in serum creatinine of 50% or more (≥1.5-fold from baseline) without any evidence of preexisting kidney disease.
Chronic kidney disease: (1) Evidence of kidney damage for ≥3 months with or without decreased glomerular filtration rate (GFR): persistent proteinuria (24-h excretion of protein >300 mg/day or spot protein-to-creatinine ratio >200 mg/g), abnormalities of urine sediment (RBCs, RBC casts, WBC), or abnormal imaging tests (hydronephrosis, cysts, masses, nephrocalcinosis or discrete stones); or (2) GFR <60 mL/min/1.73 m2 for ≥3 months, with or without kidney damage.
Acute on chronic renal failure: Increase in serum creatinine of 50% or more (≥1.5-fold) from baseline in a patient with preexisting kidney disease.
Nephrotic syndrome: Total urine protein excretion in excess of 3.5 g/day with reduced serum albumin concentration (<3 g/dL).
Nephritic syndrome: Abrupt onset of hematuria with red blood cell casts, hypertension, and edema, with or without decreased GFR.
Hepatorenal syndrome: Rise of serum creatinine to >1.5 mg/dL in patient with cirrhosis and ascites, with no improvement of serum creatinine after 48 h of diuretic withdrawal and volume expansion in the absence of shock, nephrotoxic drug treatment, and renal parenchymal disease.
STATISTICAL ANALYSES
For descriptive statistical analysis, mean, standard deviation, and frequencies were calculated. Different characteristics were represented as numbers or percentage wherever required. Statistical analysis was done by statistical software SPSS for Windows v16.0 (SPSS, Chicago, IL, USA). Comparisons between groups were performed using the Student t-test or the Mann–Whitney U test for continuous variables and the chi-square test (χ2) or the Fisher exact test for categorical data. p-Value shows the significance level (p < 0.05 or p < 0.001).
RESULTS
A total of 404 cases of cirrhosis of liver were included in this study. One hundred and seventy-eight patients were diagnosed to have different forms of renal diseases.
Demography of Patients of Cirrhosis of Liver with Renal Disorder
The mean age of cirrhotic patients was 47.7 ± 13.3 years. Maximum number of patients was in the age group of 60–69 years (27.1%) followed by 40–49 years (25.8%). Male gender constituted 77% of patients. Most common etiology of cirrhosis was chronic alcoholism (29.8%) followed by cryptogenic (25.3%) and chronic hepatitis B (24.1%). Other less common causes included chronic hepatitis C (7.3%), primary biliary cirrhosis, nonalcoholic fatty liver disease, autoimmune hepatitis, combined effects of alcoholism and hepatitis B, and Wilson's disease, in decreasing order of frequency ().
Table 1. Demography of patients with cirrhosis of liver with or without renal disorders
Clinical and Laboratory Characteristics of Patients of Cirrhosis with Renal Diseases
Of 178 cirrhotic patients with renal diseases, 15 (8.4%) had hematemesis and 29 (16.3%) had melena at the time of presentation. Pedal edema in 68 (38.2%), hemorrhoids in 6 (3.4%), hepatomegaly in 27 (15.2%), splenomegaly in 128 (71.9%), and ascites in 138 (77.5%) patients were observed. Eighteen (10.1%) patients presented with hypotension and 62 (34.8%) patients had encephalopathy at admission or developed during course of hospital stay. Persistent proteinuria was seen in 31 (17.4%) patients of which 4 (2.2%) had nephrotic-range proteinuria. Forty-nine (29.4%) cases had hematuria of which four had gross hematuria. Twenty-seven (15.2%) patients had severe anemia, 95 (53.4%) had hypoalbuminemia, 53 (29.8%) had hyponatremia, and 36 (20.2%) had leukocytosis. Upper GI endoscopy revealed the presence of esophageal varices in 147 (82.5%) patients and associated gastric and/or duodenal ulcer in 12 (6.7%) patients. Dilated portal vein was observed in 98 (55%) patients on ultrasound examination. History of diabetes mellitus and preexisting hypertension were noted in 4.5% and 6.7% cases, respectively ().
Table 2. Clinical and laboratory characteristics of cirrhotic patients with renal diseases (n = 178)
Spectrum of Renal Diseases in Patients with Cirrhosis of Liver
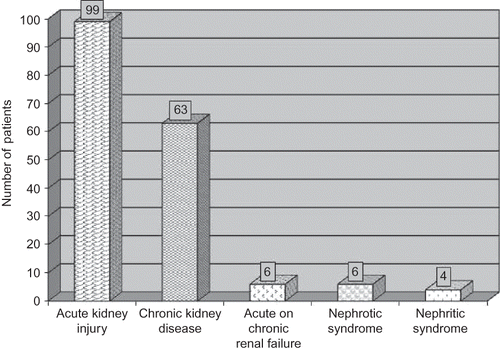
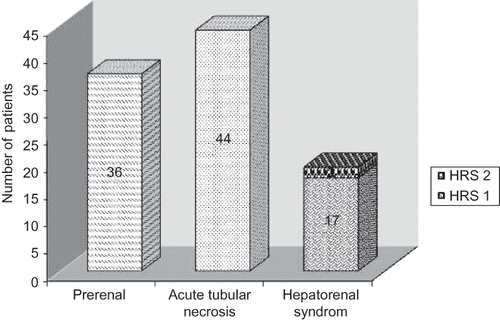
The various spectrum of clinical renal diseases in 178 patients included acute kidney injury (AKI; 99, 24.5%); CKD (63, 15.6%); and acute on chronic renal failure (6, 1.5%). In addition, nephritic and nephrotic syndromes were observed in 6 (1.5%) and 4 (1%) cases, respectively (). The differential diagnosis of acute renal failure/AKI in our cirrhotic patients were ATN (44.4%), prerenal failure (36.4%), and HRS (19.2%) (). The patients with CKD had urinary protein excretion ranged between 600 mg and 2.8 gm/24 h, with or without microscopic hematuria. Volume depletion was the main cause of acute deterioration of renal function in six patients with underlying CKD. The etiology of CKD in these six patients was unexplained (3), diabetic nephropathy (2), and multiple myeloma (1). Percutaneous renal biopsy was carried in cirrhotic patients with evidence of glomerular disease. Ten such patients (nephrotic syndrome = 4; nephritic syndrome = 6) were subjected to renal biopsy. The tissues were examined under light microscope using hematoxylin and eosin (H&E) stain. The histopathology revealed type I membrano proliferative glomerulonephritis in two patients, mesangio proliferative glomerulonephritis in five patients, diffuse endocapillary proliferative glomerulonephritis in two patients, and features of nodular glomerulosclerosis in one patient. Mesangio proliferative glomerulonephritis was the dominant lesion in patients with nephrotic syndrome, observed in three of four (75%) cases. Immunofluorescence microscopy was not carried in our study due to lack of facility. It is possible that we have missed the diagnosis of IgA nephropathy in few patients having mesangio proliferative changes.
The most common precipitating event of prerenal failure was hypovolemia in 29 (80.6%) patients. The causes of hypovolemia were upper gastrointestinal bleed in 15 (41.7%) patients, diuretics in 8 (22.2%) patients, diarrhea plus diuretics in 3 (8.3%) patients, septic shock in 2 (5.6%) patients, and lactulose-induced diarrhoea in 1 (2.8%) patient, in decreasing order of frequency. HRS was observed in 19 (19.2% of AKI) patients. Spontaneous development of HRS was observed in 9 (47.4%) patients whereas 10 (52.6%) patients had preceding history of precipitating events. Spontaneous bacterial peritonitis (SBP) was the most common precipitating event in 4 (21%) patients for HRS. The causative factors for ATN were sepsis in 27 (61.4%) cases, hypovolemia in 16 (36.4%) cases, and nephrotoxic drug in 1(2.3%) case. Sepsis was most commonly due to SBP in 15 (34.1%) patients followed by urinary tract infection (UTI) in 8 (18.2%). Sepsis was related to combination of SBP and UTI in three patients leading to ATN. Hypovolemia was mainly due to upper gastrointestinal bleed in 12 (27.3%) followed by septic shock in 4 (9.1%) patients with ATN ().
Table 3. Precipitating factors for acute kidney injury (n = 99)
Child–Pugh Score and Renal Diseases
Severity of cirrhosis of liver was classified according to modified Child–Pugh classification with scoring system of 5–15.Citation7 The relative distribution of patients with cirrhosis (n = 404) in different classes of disease were 95 in class A, 134 in class B, and 175 patients in class C categories. Fifteen patients of class A cirrhosis had renal disease (15.8%). Similarly, the incidence of renal diseases in the patients with class B and class C cirrhosis were 50% (n = 67) and 54.9% (n = 96), respectively. Renal diseases were more frequent with class B and class C cirrhosis. It is obvious that frequency of renal disease keeps on increasing with increase in severity of liver disease.
Prognostic Impact of Renal Diseases in Patients with Cirrhosis
There was no significant difference in mortality among patients with or without renal disease in class A cirrhosis (0.0% vs. 1.1%; p ≥ 0.05). Even though the mortality among class B cirrhosis patients with renal disease was higher than those without renal disease, it was not statistically significant (28.3% vs. 21.1%; p = 0.42). Patients of class C cirrhosis with renal disease had significantly higher mortality than those without renal disease (78.1% vs. 53.2%; p < 0.001) (). Thus, mortality was higher in patients with advanced cirrhosis and the presence of renal disease adds to further increase in mortality.
Table 4. Impact of renal diseases on mortality of patients with cirrhosis based on Child–Pugh classification
DISCUSSION
The etiology of renal diseases in the cirrhotic patients is varied. It may be independent of cause of cirrhosis (e.g., prerenal failure, intrinsic renal failure) or as a manifestation of the same systemic disease responsible for the liver disease or as a consequence of cirrhosis (HRS).Citation9 The first reports of renal failure in the setting of cirrhosis of liver were from Europe and the United States in the late nineteenth century.Citation10 Hecker and Sherlock in 1956 gave the first detailed description of HRS.Citation11 VesinCitation12 and Koppel et al.Citation13 later established the functional nature of this renal disease. Since then, the relationship between the liver and kidney functions has been an object of great research effort. Majority of clinical studies focused their attention on HRS, which is mostly present in advanced stages of cirrhosis. However, other forms of renal disorders in cirrhotic patients have equal importance in view of their incidence and prognostic impact in such patients.
This prospective study evaluated the incidence of renal disorders in hospitalized patients with cirrhosis of liver. The incidence of renal disorders in patients with cirrhosis of liver in our study was 44% (178/404). Garcio-Tsao et al.,Citation14 in a recent meta-analysis, reported that the incidence of renal disease was 27% in their cirrhotic patients. The reported incidence of clinical renal disease (44%) in our cirrhotic patients may not reflect the true incidence of renal disease, because we had included only indoor patients. Routine screening for evidence of renal disease in all cirrhotic patients attending the hospital (outdoor and indoor) may provide better incidence of renal disease in this population. We did not observe any significant difference in age and sex composition of cirrhotic patients with or without renal diseases.
Most common etiology of cirrhosis (n = 178) in our study was alcoholism (29.8%) followed by cryptogenic (25.3%) and hepatitis B infection (24.1%). This is in agreement with the study of KumarCitation15 in cirrhotic patients from Bihar (India). He reported alcoholism as the most common cause of cirrhosis of liver. However, Joshi et al.Citation16 and Sarin et al.Citation17 had reported that hepatitis B infection was the most common cause of cirrhosis of liver in northern and western parts of India, respectively. The disparity can be explained by the fact that the National Family Health Survey-3 showed a higher prevalence of alcohol consumption among men in eastern part than in the western part of India. Alcohol and Drugs Information Centre- (ADIC-) India revealed that consumption of alcohol among people with younger age has increased in Kerala (India).Citation18 This may explain higher incidence of alcoholic cirrhosis in certain parts of India.
The spectrum of clinical renal diseases in our study was AKI (99, 24.5%); CKD (63, 15.6%); acute on chronic renal failure (6, 1.5%); nephritic syndrome (6, 1.5%); and nephrotic syndrome (4, 1%). ATN was the most common cause of AKI (44.4%). Moreau et al.Citation4 in their study had reported that ATN was the most common cause of AKI (148/381; 41.7%). Incidence of HRS in our study was 19.2% of the total cases of AKI and majority (17/19; 89.5%) were of type 1 HRS. This is in agreement with study of Moreau et al.,Citation4 who reported that incidence of HRS was 20% in their cirrhotic patients. HRS was of spontaneous origin in 47.4% cases, which differs from that observed by PapperCitation19 (24%). Most common precipitating factor for HRS was SBP (40%), followed by upper GI bleed (20%). Similar observations were made by Watt et al.,Citation20 who reported bacterial infection as the most common precipitating factor (48%) followed by gastrointestinal bleed (33%).
Overall prevalence of CKD in general population in different studies varies from 7.2% to 13.7%.Citation21–24 The observed prevalence of CKD in our cirrhotic patients was 15.6%. The higher incidence of CKD in our study (15.6%) may be due to common denominator causing cirrhosis of liver and CKD, for example, viral infection (hepatitis B and C) is known to cause both. Acute deterioration of CKD was observed in 6 (1.5%) patients. All of them were due to volume depletion and their condition improved after institution of effective volume replacement. We observed a proportionate increase in the incidence of renal disease with increase in the severity of liver disease as determined by Child–Pugh classification. The incidence of renal diseases was 15.7% in class A, 50% in class B, and 54.8% in class C of Child–Pugh classification. Our findings were supported by Attia et al.,Citation5 where the authors have found the incidence of renal disease to be 6.7%, 13.5%, and 33.9% in patients with Child–Pugh class A, B, and C cirrhosis, respectively. Furthermore, analysis of impact of renal disease on mortality of cirrhotic patients with respect to severity of liver disease (Child–Pugh class B and C) showed that renal disease significantly increases the mortality in patients with class C cirrhosis (78.1% vs. 53.2%; p < 0.001). Therefore, renal disease can be considered as an independent prognostic indicator in patients with class C cirrhosis.
In summary, clinical renal disease are common in cirrhotic patients and AKI are the commonest type of kidney disease. The reversible form of AKI (prerenal and ATN) was observed in the majority (80.8%) of patients. The cirrhotic patients had higher incidence of CKD compared with general population (15.6% vs. 10%). We suggest that routine screening of all patients with liver diseases will help us in determining the true incidence of kidney diseases in cirrhotic population.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bacon BR. Cirrhosis and its complications. In: Fauci AS, ed. Harrison's Principles of Internal Medicine. 17th ed., Vol. 2. New York: McGraw Hill; 2008:1971–1980.
- Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: Perspectives in the Age of MELD. Hepatology. 2003;37:233–243.
- Howard CS, Teitelbaum I. Renal replacement therapy in patients with chronic liver disease. Semin Dial. 2005;18(3):212–216.
- Moreau R, Durand F, Poynard T, Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: A retrospective multicenter study. Gastroenterology. 2002;122:923–930.
- Attia KA, N'dri Yoman AT, Mahassadi AK, Impact of renal failure on survival of African patients with cirrhosis. Saudi J Kidney Dis Transpl. 2008;19(4):587–592.
- Sherlock S, Doodley J. Diseases of the Liver and Biliary System. 11th ed., London: Blackwell Publishing Ltd.; 2002:365–380.
- Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649.
- Moore KP, Wong F, Gines P, The management of ascites in cirrhosis: Report on consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266.
- Pham PTT, Pham PCT, Rastogi A, Wilkinson AH. Review article: Current management of renal dysfunction in the cirrhotic patient. Aliment Pharmacol. Ther. 2005;21(8):949–961.
- Ginès A, Escorsell A, Ginès P, Incidence, predictive factors, and prognosis of hepatorenal syndrome in cirrhosis. Gastroenterology. 1993;105:229–236.
- Hecker R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956;268:1121–1125.
- Vesin P. Late functional renal failure in cirrhosis with ascites: Pathophysiology, diagnosis and treatment. In: Martini GA, Sherlock S, eds. Aktuelle Probleme der Hepatologie. Stuttgart: George Thieme Verlag; 1962:98.
- Koppel MH, Coburn JW, Mims MM, Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. N Engl J Med. 1969;280:1367.
- Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48(6):2064–2077.
- Kumar T. Incidence of cirrhosis caused by hepatitis b virus in different sex and age groups in Bihar. J MGIMS. 2006;11(i):52–54.
- Joshi PH. Chronic hepatitis ‘B’. Bombay Hosp J. 1996;3804(712):701.
- Sarin SK, Guptan RC, Banerjee K, Khandekar P. Low prevalence of hepatitis C viral infection in patients with non alcoholic chronic liver disease in India. JAPI 1996;44(1):243–245.
- Edayaranmula JJ. Drinking patterns in Kerala, ADIC-INDIA. 2007. Available at: http://www.adicindia.org. Accessed October 9, 2009.
- Papper S. The hepatorenal syndrome. In: Epstein M, ed. The Kidney in Liver Disease. 2nd ed. New York: Elsevier Biomedical;1983:87–106.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: Diagnostic accuracy, clinical features and outcome in a tertiary care centre. Am J Gastroenterol. 2002;97:2046–2050.
- Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health. 2008;8:117.
- Anothaisintawee T, Rattanasiri S, Ingsathit A, Attia J, Thakkinstian A. Prevalence of chronic kidney disease: A systematic review and meta-analysis. Clin Nephrol. 2009;71(3):244–254.
- Kim S, Lim CS, Han DC, The prevalence of chronic kidney disease and the associated factors to CKD in urban Korea: A population-based cross sectional epidemiologic study. J Korean Med Sci. 2009;24(Suppl. 1):S11–S21.
- Levey AS, Stevens LA, Schmid CH, A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.