Abstract
Sclerosing peritonitis (SP) is a rare but serious complication of peritoneal dialysis (PD), characterized by a fibrous peritoneal thickening. The etiology of this condition remains unknown but is likely to be multifactorial. Patients with SP almost invariably develop ultrafiltration and clearance failure. Although a number of pharmacologic drug treatment options have been tried with various results, surgical treatment and cessation of PD are almost always necessary and transfer to hemodialysis is the only practical option. Despite some evidence supporting the recovery of gastrointestinal function after renal transplantation in such patients, SP may very rarely appear much later after the cessation of PD and even after renal transplantation. We report an interesting case of a former PD patient who 2 years after renal transplantation presented with abdominal discomfort, vomiting, and malnutrition due to SP. Despite the initial conservative treatment, the symptoms persisted and a surgical treatment was decided upon. After that the patient recovered with no further complications. Although the appearance of SP after renal transplantation is extremely rare, it must be included in the differential diagnosis of every case of unexplained malnutrition and abdominal obstruction in a patient with a PD history.
INTRODUCTION
Sclerosing peritonitis (SP) is an inflammatory process affecting the peritoneum diffusely and constitutes a rare but very important cause of morbidity and mortality in patients on peritoneal dialysis (PD). Although a true estimate of the relative frequency of the SP is difficult to make, an overall prevalence of 0.7% has been described.Citation1
The pathogenesis of this condition still remains uncertain. Some predisposing factors include the use of chlorhexidine in alcohol-sterilizing sprays, acetate dialysate, hyperosmolar dialysate, episodes of infective peritonitis, and a prolonged duration time on PD.Citation1,Citation2 SP includes a spectrum of changes ranging from opacification, through tanned or thickened peritoneum, to sclerosing encapsulating peritonitis (SEP). SEP is a diffuse process involving the formation of new fibrous tissue that cover, bind, and constrict the viscera, thereby compromising the motility of the bowel.Citation2
A progressive reduction of peritoneal permeability results in the development of ultrafiltration failure. Abdominal discomfort with accompanying malnutrition and evidence of complete or partial bowel obstruction are almost always present.Citation1,Citation2
A number of pharmacologic drug treatment options have been tried with various results and some reports support the recovery of gastrointestinal function in patients with SP after successful renal transplantation.Citation3
We describe an interesting case of a patient with a previous PD medical history who 2 years after a successful renal transplantation presented with severe SEP. The diagnosis, management, and clinical course of the patient are discussed.
CASE PRESENTATION
A 55-year-old female patient with end-stage renal disease, secondary to medullary cystic kidney disease, was on continuous ambulatory peritoneal dialysis (CAPD) for 5 years. She received a cadaveric renal allograft in October 2005. Her past medical history included arterial hypertension, chronic hyperuricemia, and one episode of infective peritonitis due to CAPD. The patient was given induction therapy with anti-CD25 monoclonal antibodies and an initial triple immunosuppressive regimen with prednisolone, cyclosporine, and mycophenolate mofetil. Soon after transplantation, the administration of mycophenolate mofetil was stopped because the patient developed severe anemia. Several months later, cyclosporine was also switched to tacrolimus due to gum hyperplasia; but again, this time due to severe hair loss, tacrolimus was replaced by sirolimus with no further side effects. Her renal graft function was excellent with an estimated glomerular filtration rate of 58 mL/min.
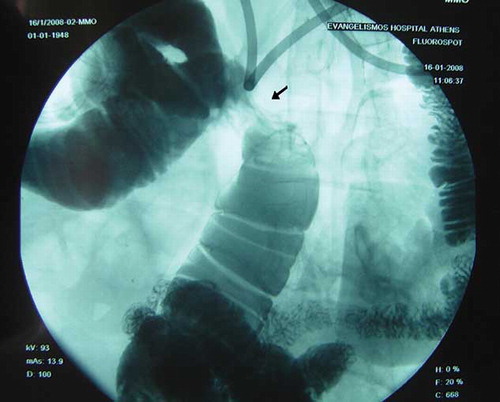
In December 2007, she presented with worsening abdominal discomfort, vomiting, and weight loss. Clinical examination revealed a suffering person with abdominal distension and signs of partial small bowel obstruction. Plain abdominal radiograph was indicative of intestinal obstruction. This was also confirmed by abdominal computed tomography scan that showed enlarged small bowel loops and multiple air-fluid levels but with no thickening or calcification of the peritoneum (). A small intestine transit study (enteroclysis) demonstrated decreased bowel motility and strictures (). Colonoscopy was unremarkable. Laboratory evaluation was normal except for low albumin levels. Based on the CAPD history, a diagnosis of SP was suspected. The patient was set initially on total parenteral nutrition (TPN) with gradual improvement. Two weeks later, oral nutrition was attempted gradually and allowed TPN to be discontinued. The patient was discharged in a better condition but 2 months later the symptoms relapsed and she was hospitalized again.
Figure 1. Abdominal computed tomography scan. Enlarged small bowel loop caused by obstruction. No peritoneal thickening was demonstrated.
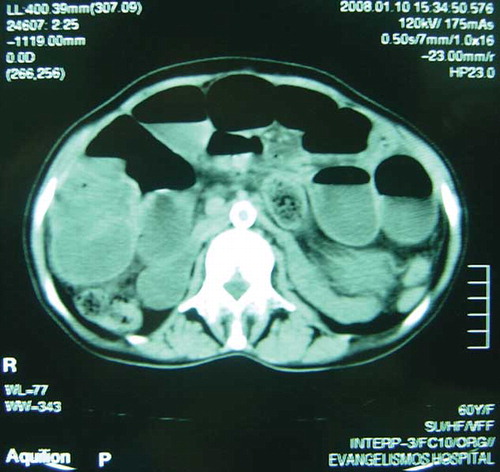
Figure 2. Small intestine transit study. A marked stricture is shown in the middle of the picture (arrow).
A surgical approach was decided upon at this point. The operative findings were those of SP with dense fibrotic adhesions encasing her small bowel especially at the distal loops of the ileum. Lysis of the fibrotic tissue and release of the intestinal loops was performed but an extended right hemicolectomy was inevitable due to severe ileum involvement. Histology revealed a terminal ileum with marked peritoneal thickening and fibrous tissue deposition (). The postoperative period was uncomplicated. The patient improved and was discharged with instructions. Two years after surgery, the patient is in a perfect clinical condition with a 7-kg weight gain and a stable renal function.
DISCUSSION
Although the prevalence of SP is quite low, it greatly increases with the duration of PD.Citation1 With the duration of PD being so important, other factors apart from peritonitis must be considered. Glucose-based dialysate may be an important factor. The formation of advanced glycation end-products, as a result of glucose degradation by heat sterilization of glucose-based PD fluids, has been described more recently as a possible pathogenetic pathway. The accumulation of advanced glycation end-products is observed in mesothelial and submesothelial layers in CAPD patients, accompanied by the enhanced expression of various growth factors and peritoneal thickening.Citation4
The clinical features are variable and include abdominal pain, nausea, vomiting, weight loss, and complete or partial bowel obstruction.Citation1 Loss of ultrafiltration is another important feature with many patients having to increase the daily dialysate volume or the tonicity of the fluid or to combine a regular hemodialysis session with CAPD.Citation5 Diagnosis is based on surgical findings of encapsulated, tanned, or thickened peritoneum.Citation1 Contrast-enhanced computed tomography may reveal peritoneal thickening and calcification, loculated fluid collections, and bowel wall thickening.Citation6
Treatment options are variable and include steroid administration, either alone or in combination with azathioprine.Citation1 Tamoxifen, a nonsteroidal antiestrogen, has been tried with encouraging results.Citation7 More recently, mammalian Target Of Rapamycin (mTOR) inhibitors have also been tried in a rat model with promising results.Citation8 However, neither the ideal dose of the immunosuppressive therapy nor its duration for effective SP treatment is known. In more severe cases, TPN may be necessary.Citation3 If intestinal obstruction persists and symptoms remain, laparotomy and lysis of adhesions should be considered. Surgery is, however, usually difficult. Resection and primary intestinal anastomosis should be avoided because of the high incidence of anastomotic failure.Citation9
Some reports support the recovery of gastrointestinal function in patients with SP after successful renal transplantation, suggesting that this condition may improve because of the anti-inflammatory effects of the immunosuppression or the reversal of the uremic state.Citation3 On the other hand, there are also a few reports of SP presenting after renal transplantation. Bowers et al. described three such cases. In two of the patients, symptoms came on just within few months after transplantation and in the third case the onset was 1 year posttransplantation.Citation10
Our patient was on CAPD for 5 years. She was in excellent nutrition state at the time of transplantation with no symptoms indicating SP. Furthermore, she had never manifested any evidence of ultrafiltration failure. The symptoms appeared 2 years after transplantation and while she was on maintenance immunosuppressive therapy with prednisolone 5 mg/d and sirolimus 2 mg/d. Despite the fact that there have already been some reports of SP presenting after renal transplantation, this is the first case to our knowledge of severe SEP presenting so late, that is, 26 months after successful transplantation. Moreover, what is interesting in this case is that SP appeared despite the immunosuppression with two drugs (steroids and mTOR inhibitors) that have already been used for the treatment of the syndrome.
In conclusion, this case suggests that SP is a process that can persist or even progress for a long time after the cessation of PD, and this might be related to loss of lavage and derangement of the balance between fibrin exudation on the peritoneal surface and its removal.Citation2 Moreover, the usual maintenance immunosuppression dose is probably not efficient in reversing the pathologic process and even after successful renal transplantation, SP must be suspected in every former PD patient presenting with malnutrition and abdominal obstruction.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rigby RJ, Hawley CM. Sclerosing peritonitis: The experience in Australia. Nephrol Dial Transplant. 1998;13:154–159.
- Dobbie JW. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit Dial Int. 1992;12:14–27.
- Haweley CM, Wall DR, Johnson DW Recovery of gastrointestinal function after renal transplantation in a patient with sclerosing peritonitis secondary to continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1995;26:658–661.
- Nakamura S, Niwa T. Advanced glycation end-products and peritoneal sclerosis. Semin Nephrol. 2004;24:502–505.
- Afthentopoulos IE, Passadakis P, Oreopoulos DG, Bargman J. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: One center's experience and review of the literature. Adv Ren Replace Ther. 1998;5:157–167.
- George C, Al-Zwae K, Nair S, Cast JEI. Computed tomography appearances of sclerosing encapsulating peritonitis. Clin Radiol. 2007;62:732–737.
- Eltoum MA, Wright S, Atchley J, Mason JC. Four consecutive cases of peritoneal dialysis-related encapsulating peritoneal sclerosis treated successfully with tamoxifen. Perit Dial Int. 2006;26:203–206.
- Duman S, Bozkurt D, Sipahi S Effects of everolimus as an antiproliferative agent on regression of encapsulating peritoneal sclerosis in a rat model. Adv Perit Dial. 2008;24:104–110.
- Kittur DS, Korpe SW, Raytch RE, Smith GW. Surgical aspects of sclerosing encapsulating peritonitis. Arch Surg. 1990;125:1626–1628.
- Bowers V, Ackerman JR, Richardson R, Carey L. Sclerosing peritonitis. Clin Transplant. 1994;8:369–372.
