Abstract
The hallmark of IgA nephropathy (IgAN) is the mesangial deposits of polymeric IgA. However, the source of IgA1 and the mechanism of deposition of IgA1 in the mesangium remain unknown. To better understand its pathogenesis, we investigated the expression of CD19+CD5+B cells and IgA1-positive cells in the tonsils of IgAN patients. Immunofluorescence was used to visualize the locations of CD19+CD5+B cells and IgA1-positive cells in the tonsils. In this study, it was demonstrated that CD19+CD5+B cells are usually found in germinal centers and in the capsule covering the upper parts of the nodules of lymphoid tissue (cap of the nodule). The expression of IgA1-positive cells in tonsil tissue can be seen in the cap of the nodule and subepithelial tissue. There is a significant relationship between IgA1 and CD19+CD5+B cells. The level of CD19+CD5+B cells is positively correlated to the severity of renal pathological changes. These findings suggest that CD19+CD5+B cells in the tonsils could have an impact on the pathogenesis of IgAN.
INTRODUCTION
IgA nephropathy (IgAN) is the most common form of progressive glomerulonephritis, characterized by mesangial IgA1 deposits, although its pathogenesis is unclear.Citation1 The course of IgAN was initially considered benign, but it is now known that progression to end-stage renal failure often occurs over a period of 20 years. It is also associated with molecular aberrancy and/or defect of IgA1 in mucosal and tonsillar immunity. It is recognized as a tonsil-related disease because it often gets worse after and/or during acute tonsillitis and the disease progression is often arrested by tonsillectomy.Citation2,Citation3
The CD19+CD5+B cells (B-1a) are derived from precursors of the fetal liver and omentum and are located in the peritoneal and pleural cavities, whereas few, if any, are found in the spleen and lymph nodes in the normal adult. They are maintained by self-regeneration in the peritoneum and produce mostly low-affinity IgM class antibodies in a T-cell-independent manner, which, as a subtype of B1 cells, is an important producer of IgA.Citation4,Citation5 It is likely that IgA1 antibody-producing cells are present in mucosal tissuesCitation6 and may present as a marker for a dysregulated B-cell development driven by a greater B-cell receptor signaling.Citation7,Citation8 IgAN is a glomerular disease that is likely a disease of overexpression of B-cell activation factor.Citation9
Are CD19+CD5+B cells involved in the pathogenesis of IgAN
The immune responses of CD19+CD5+B cells in the tonsillar germinal center (GC) were documented in a single report and could thus have an impact on the pathogenesis of IgAN.Citation10 However, a study on the numbers of these cells in different anatomic locations and relationships of the cells in patients with IgAN has not yet been conducted.
In this study, we investigated the expression of CD19+CD5+B cells and IgA1-positive cells in tonsillar tissues of eight IgAN patients and eight patients with chronic tonsillitis but without renal disease. We also investigated the pathological changes in IgAN patients to further clarify the relationship between the percentage of tonsillar B-1a cells and the Lee classification of IgAN.
MATERIALS AND METHODS
Patients
Tonsillar tissue obtained from eight IgAN patients (6–52 years of age, mean = 32.2 years; five males and three females) treated by tonsillectomy in our clinic was used in this study, and tonsillar tissue from eight age-matched patients with chronic tonsillitis without IgAN (18–49 years of age, mean = 31.5 years; four males and four females) was used as controls. All IgAN patients were diagnosed with IgAN by renal biopsy, and all patients with IgAN had microhematuria, five of them had proteinuria as well (mean = 1.02 g/day urine protein excretion). The HS Lee grading systemCitation11 was used to test for pathological changes in the patients with IgAN.
The patients had no other diseases and were not on any antibiotics or immunosuppressive drugs in the 3 months before the study. We were consulted for tonsillectomies for the treatment of IgAN in these patients. In all the cases, informed consent was sought and ethical permission was granted.
Reagents
The following antihuman immunoglobulins were used for immunofluorescence analyses: fluorescein isothiocyanate (FITC)-conjugated monoclonal mouse antihuman IgA1 antibody was purchased from SBA (Peking, China). FITC-conjugated CD5 polyclonal rabbit anti-human IgG antibody, CD19 polyclonal goat anti-human IgG antibody, goat anti- rabbit Monoclonal IgG Antibody (FITC) and Rhodamine B isothiocyanate (RBITC) -conjugated goat anti-rabbit Monoclonal IgG Antibody were obtained from Biosynthesis Biotechnology Co. LTD (Peking, China).” 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) was obtained from Roche (Basel, Switzerland). Nuclear fast red was obtained from GENMED (Boston, MA, USA).
Immunofluorescence
Tissue preparations were embedded in paraffin and cut in 4-mm thick sections. Before visualization of proteins, the sections were treated with xylene to remove the paraffin and rehydrated using ethanol. To facilitate the binding of the antibodies, the sections were treated with boiling citric acid buffer for 3 min in a microwave oven. After cooling to room temperature, unimmunized sheep serum was used for 10 min to block nonspecific binding. Primary CD19 (1:40 dilution) was incubated for 1 hour at 37°C. Goat anti-rabbit monoclonal IgG antibody (RBITC) was used for 30 min at 37°C. Subsequently, rabbit antibodies against CD5 (1:40 dilution) were incubated for 1 hour at 37°C. Goat anti-rabbit monoclonal IgG antibody (FITC) was used for 30 min at 37°C. Slides were then stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) for 3 min, a fluorescent blue nuclear indole chromatin stain, and mounted in glycerol. Phosphate-buffered saline was used as isotype negative control slides. The procedures for IgA1-positive cells immunofluorescence were the same as above, except that the primary antibody was IgA1–FITC and the nuclear stain was nuclear fast red. A phosphate-buffered saline supplement was used for all labeling and washing steps. Observations were done using a confocal laser scanning microscope. In 500 nm laser, CD19+cell is red; in 489 nm laser, CD5+cell is green; but under a confocal microscope, CD19+CD5+B cell is yellow; and with fluorescence microscope, the IgA1-positive cell is green.
Statistical analysis
Image analysis with Image-Pro plus 6.0 (Media Cybernetics, Inc., USA)analysis software was used to semiquantitatively determine the percentage of CD19+CD5+ B cells and IgA1 positive cells. All the data were replaced by median and quartile intervals. The variances were nonhomogenous; hence, Wilcoxon signed-rank test was used to determine statistical differences for paired comparisons, and rank correlation was used for correlation of different variables. p-Values <0.05 were considered statistically significant. The SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for data analysis.
RESULTS
Expression of CD19+CD5+B Cells in Tonsillar Tissue of IgAN Patients
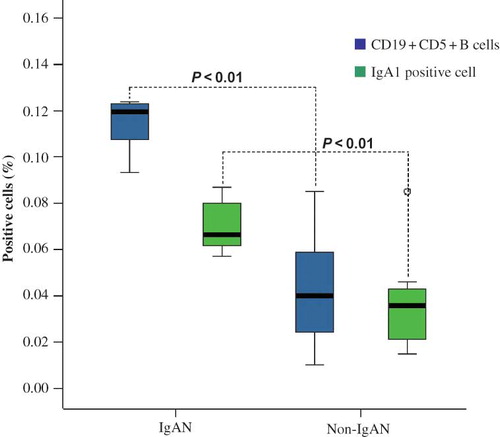
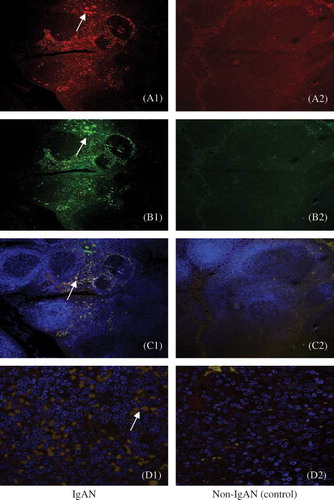
In tonsils of IgAN patients, CD19+CD5+B cells were found mostly in GCs and in the cap of the nodule. Around the internodal zone and the capillaries, very few CD19+CD5+B cells can be detected. The controls had few positive cells ().
Figure 1. Expression of CD19+CD5+B cells in GC and cap covering the top of the nodule of lymphoid tissue. In IgAN, there is more expression, whereas little to no staining was detected with the control. Arrows indicate positive cell staining (A) RBITC-conjugated CD19 (red); (B) FITC-conjugated CD5 (green); (C) double staining of CD19+CD5+B cells (yellow) on the surface of nodules of lymphoid tissue, confocal laser, ×100; (D) double staining of CD19+CD5+B cells in germinal center, confocal laser, ×630).
Expression of IgA1-Positive Cells in Tonsillar Tissue of IgAN Patients
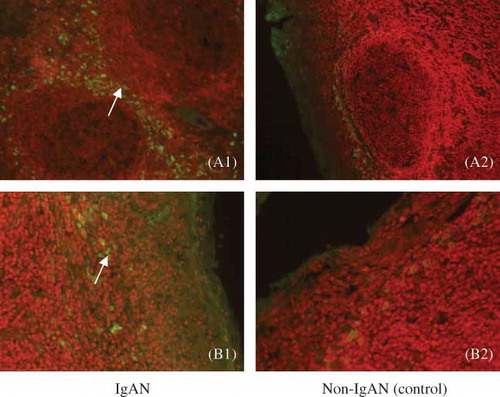
Expression of IgA1-positive cells exists mainly in the broader areas of the epithelium and subepithelium, and cap of the nodule. Few can be found in GCs and diffuse lymph nodes. Compared with the controls, the expression of IgA1-positive cells was significantly increased in the tonsillar tissue of IgAN patients ().
Figure 2. Expression of IgA1-positive cells in tonsillar tissue can be seen in cap covering the top of the nodule of lymphoid tissue and the subepithelial tissue (green). In IgAN, there is more expression, whereas little to no staining was detected with the control. Arrows indicate positive cell staining in (A) cap covering the top of the nodule of lymphoid tissue (fluorescence microscope, ×100) and (B) subepithelial tissue (fluorescence microscope, ×200).
Difference between CD19+CD5+B Cells and IgA1-Positive Cells in Tonsillar Tissue of IgAN and Non-IgAN Patients
The average percentages of CD19+CD5+B cells and IgA1-positive cells in the tonsils of IgAN and non-IgAN patients were calculated using the Wilcoxon signed-rank test. The difference was statistically significant (p < 0.05; ).
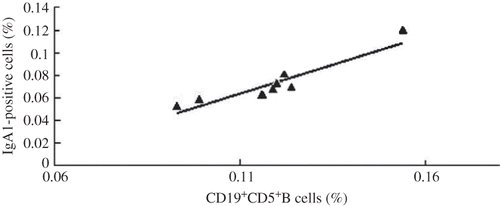
Correlation between CD19+CD5+B Cells and IgA1-Positive Cells
Summarizing the rank correlations between CD19+CD5+B cells and IgA1-positive cells, we found a close correlation between them (r = 0.778, p = 0.023; ).
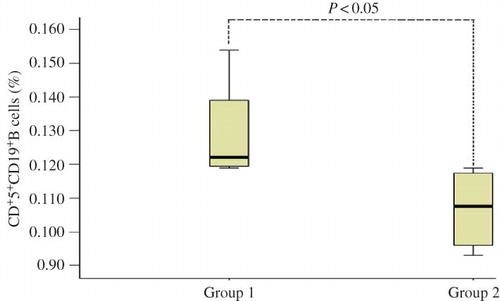
Correlation between CD19+CD5+B Cells and Renal Pathologic Changes in IgAN Patients’ Tonsils
The histological changes of kidney biopsies in patients with IgAN were graded by Lee classification.Citation10 The pathological changes of four IgAN patients were graded between IV and V, and their average percentage of CD19+CD5+B cells (group 1) was 0.120. For renal pathological changes (group 2), Lee's grade I–III was dominant, with the average percentage being 0.107. The Wilcoxon signed-rank test showed that the difference between the two groups was statistically significant (p < 0.05; ).
DISCUSSION
IgAN, which features polymeric IgA-dominant depositions in the mesangium, is the most common form of primary glomerulonephritis worldwide. The clinical and pathological features of IgA antibodies are important effectors of mucosal protection. The two main subpopulations of B cells, B1 and B2, are capable of producing IgA. Differences in their anatomic locations, sensitivity to activating signals, and cytokine and receptor expression may determine the relative contribution of B1 versus B2 cells to IgA production at a specific site.Citation12–16 The surface phenotypes of B-1a cells are CD19+ and CD5+, which are distinguished from the surface phenotypes of B-2 cells (CD19+ and CD5−). They usually do not take part in the GC reaction. However, in autoimmune reaction-prone individuals, CD19+CD5+B cells increase in number in the GC and have been suggested to be involved in the generation of self-reactive antibodies. It has been recently reported that CD19+CD5+B cells are the main IgA-producing cells in mucosal tissues.Citation17
GC is a histologically important area in the tonsils and comprises mostly proliferating B cells, along with follicular dendritic cells, tingle body macrophages, and a small number of T cells. Antigen-specific resting B cells are activated in T-cell-rich areas outside the GC of secondary lymphoid organs and give rise to IgM-secreting plasma cells (PCs) and B-cell blasts that colonize the primary follicles. The subsequent GC reaction is initiated by the rapid proliferation of a few B-cell blasts in association with follicular dendritic cells, whereas the remaining B cells follow the differentiation pathway from centroblast to centrocytes and then to either PCs or memory B cells. During these processes, somatic hypermutation, positive selection, and differentiation of selected GC B cells occur. GCs are thus dynamic microenvironments in which not only antigen-specific B cells are selected for high-affinity immunoglobulin but self-reactive and antigen-unresponsive B cells are destroyed by apoptosis. Nossal et al.Citation18 suggested that to avoid autoimmunity reactions, any newly formed self-reactive B cells are eliminated within the GC by apoptosis.
In our study, CD19+CD5+B cells are found mainly in GC and the surface of nodules of lymphoid tissue, which were identified with Kodama's study,Citation6 whereas the expression of IgA1-positive cells in tonsillar tissue can be seen on the surface of nodules of lymphoid tissue and the subepithelial tissue, which was identified with Itoh's.Citation19 From the position of IgA1-positive cells and CD19+CD5+B cells, we can have a hypothesis about a pathway of migrating of CD19+CD5+B cells in the tonsils of IgAN patients: stimulated by antigen in GC and/or the surface of nodules of lymphoid tissue, CD19+CD5+B cells are activated, somatic hypermutated, antibody gene rearranged, class switch recombinated, and translated into IgA1-secreting PCs. Around the surface of nodules of lymphoid tissue and the subepithelial tissue, they secrete IgA1 into blood.
In humans, elevated numbers of CD19+CD5+B cells have been reported in patients with Sjögren's syndrome, rheumatoid arthritis, and chronic lymphocytic leukemia.Citation20,Citation21 In mice, increased numbers of CD19+CD5+B cells are observed in a number of naturally occurring and genetically manipulated strains that develop lupus-like disease manifestations.Citation22 He Yuling et al.Citation23 also reported that the majority of patients with IgAN were responsive to corticosteroid and immunosuppressive drug treatments, and clinical improvement correlates with reductions in CD19+CD5+B cells. In this study, the average percentages of CD19+CD5+B cells and IgA1-positive cells in the tonsils of IgAN and non-IgAN patients were significantly correlated; at the same time, the percentage of CD19+CD5+B cells is also positively related to the severity of renal pathological changes, similar to He Yuling'sCitation23 findings. Therefore, we can also draw a conclusion that the role of CD19+CD5+B cells in IgAN may be important in the pathogenesis of IgAN.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–748.
- Miura N, Imai H, Kikuchi S, Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: A nation-wide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol. 2009;13:460–466.
- Fujinaga S, Ohtomo Y, Hirano D, Low-dose pulse methylprednisolone followed by short-term combination therapy and tonsillectomy for childhood IgA nephropathy. Pediatr Nephrol. 2010;25:563–564.
- Kataoka K, Fujihashi K, Sekine S, Nasal cholera toxin elicits IL-5 and IL-5 receptor alpha-chain expressing B-1a B cells for innate mucosal IgA antibody responses. J Immunol. 2007;178:6058–6065.
- Fagarasan S, Honjo T. T-independent immune response: New aspects of B cell biology. Science. 2000;290:89–92.
- Kodama S, Suzuki M, Arita M, Mogi G. Increase in tonsillar germinal centre B-1 cell numbers in IgA nephropathy (IgAN) patients and reduced susceptibility to Fas-mediated apoptosis. Clin Exp Immunol. 2001 February; 123(2): 301–308.
- Byian Duana and Laurence Morel. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–408.
- Richard R Hardy. B- 1 B cells: Development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555.
- McCarthy DD, Chiu S, Gao Y, Summers-deLuca LE, Gommerman JL. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell Immunol. 2006;241:85–94.
- Kodama S, Suzuki M, Arita M, Mogi G. Increase in tonsillar germinal centre B-1 cell numbers in IgA nephropathy (IgAN) patients and reduced susceptibility to Fas-mediated apoptosis. Clin Exp Immunol. 2001;123:301–308.
- Lee HS, Lee MS, Lee SM, Histological grading of IgA nephropathy predicting renal outcome: Revisiting H.S. Lee's glomerular grading system. Nephrol Dial Transplant. 2005;20(2):342–348.
- Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433.
- Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12:346–354.
- Su I, Tarakhovsky A. B-1 cells: Orthodox or conformist? Curr Opin Immunol. 2000;12:191–194.
- Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300.
- Haas KM, Poe JC, Steeber DA, Tedder TF. B- 1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18.
- Hiroi T, Yanagita M, Iijima H, Deficiency of IL-5 receptor α-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosa-associated tissues. J Immunol. 1999;162:821–828.
- Nossal GJV, Karvelas M, Pulendran B. Soluble antigen profoundly reduces memory B-cell numbers even when given after challenge immunization. Proc Natl Acad Sci USA. 1993;90:3088–3094.
- Itoh A. Disordered balance of IgA subclass production in the tonsils of some IgA nephropathy patients. J Nephrol. 2005;18(5):575–581.
- Cioca DP. Apoptosis induction by hypercross-linking of the surface antigen CD5 with anti-CD5 monoclonal antibodies in B cell chronic lymphocytic leukemia. Leukemia. 2002;16(3):335.
- Pers JO. CD5-induced apoptosis of B cells in some patients with chronic lymphocytic leukemia. Leukemia. 2002;16(1):44.
- Macpherson A, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226.
- Yuling H, Ruijing X, Xiang J, CD19+CD5+B cells in primary IgA nephropathy. J Am Soc Nephrol. 2008;19(11):2130–2139.