Abstract
Background: Urine beta2-microglobulin (beta2-MG) was mainly used as a tubular marker of Balkan endemic nephropathy (BEN) but recently alpha1-microglobulin (alpha1-MG) was proposed for the diagnosis of BEN. In this study, the potential of urine beta2-MG, alpha1-MG, albumin, and total protein in the differentiation of BEN from healthy persons and patients with glomerulonephritis (GN) and nephrosclerosis (NS) was examined. Methods: This study involved 47 patients with BEN, 36 with GN, 11 with NS, 30 healthy subjects from BEN families, and 46 healthy subjects from non-BEN families. Results: In BEN patients area under the curve (AUC) for urine beta2-MG (0.828) and alpha1-MG (0.782) was higher than for urine albumin (0.740), but in GN patients AUC for urine protein (0.854) and albumin (0.872) was significantly higher than for the two low molecular weight proteins. AUC for all four urinary markers in NS patients was significantly lower than in BEN patients, ranging between 500 and 595. Median urine beta2-MG excretion in BEN patients was 17.5 times higher than in GN patients and 18.3 times higher than in controls; median alpha1-MG excretion was higher only 3.0 and 2.25 times, respectively. In the differentiation of BEN from healthy controls, beta2-MG had higher sensitivity and specificity at the cutoff levels (p < 0.001) than alpha1-MG (p < 0.05). In the differentiation of BEN from GN, beta2-MG was the best marker. Conclusion: All four urinary markers can be used for the differential diagnosis of BEN, beta2-MG being the best. Like in aristolochic acid nephropathy, beta2-MG seems to be an early marker of tubular damage in BEN.
INTRODUCTION
Balkan endemic nephropathy (BEN) is a chronic tubulointerstitial nephropathy that has been attracting the attention of numerous investigators for more than 50 years. Recently, methods for screening and diagnosis of BEN have become one of the main objectives of interest. The most widely used Danilovic's diagnostic criteria have been shown as insufficiently sensitive and specific.Citation1 Tubular proteinuria is not involved among these criteria although it presents as a characteristic of BEN. This was evidenced as early as 1966 with electrophoresis on paper and gel,Citation2 and subsequently increased excretion of low molecular weight proteins such as beta2-microglobulin (beta2-MG), lysozyme, ribonuclease, light chains of immunoglobulins, and retinol-binding protein was confirmed.Citation3–5 Hall et al.Citation2,Citation5 were the first who indicated tubular proteinuria as an early sign of BEN. Stefanović et al.Citation6 involved low molecular weight proteinuria among the diagnostic criteria and subsequently reported increased beta2-MG excretion not only in patients with BEN but also in clinically healthy relatives and children from BEN families.Citation7,Citation8 It was suggested that beta2-MG can be used not only as an early sign of tubular injury and diagnostic marker of BEN but also as a prognostic factor.Citation9,Citation10 However, the instability of beta2-MG, which is degraded in the urine with pH below 6, directed some authors to use alpha1-microglobulin (alpha1-MG), which is stable in the urine over the physiological pH range, in diagnostic procedure of BEN.Citation11,Citation12 Nevertheless, there are much more studies that use beta2-MG than alpha1-MG in the diagnosis of BEN, and neither one comparative study of these two low molecular weight proteins as markers of BEN.
In this study, urine excretion of beta2-MG, alpha1-MG, albumin, and total protein was examined in patients with BEN, glomerulonephritis (GN), nephrosclerosis (NS), and healthy persons with the aim to compare their diagnostic potential to differentiate BEN from healthy persons and patients with GN and NS.
PATIENTS AND METHODS
This study involved 170 persons: 47 patients with BEN, 36 with GN, 11 with NS, 30 clinically healthy subjects from BEN families, and 46 healthy subjects from non-BEN families. Patients with GN and NS were recruited consecutively in the Outpatient Department of the Clinic of Nephrology, Clinical Center of Serbia, Belgrade. Patients with BEN, healthy subjects from BEN families, and healthy subjects from non-BEN families were registered during the screening studies carried out in endemic villages in the region of the South Morava River and the Kolubara River. These subjects were invited to examination in the outpatient departments of the Clinical Center of Niš and the Institute of Endemic Nephropathy in Lazarevac. All persons included in this study were subjected to objective examination and their blood and urine samples were taken for laboratory examinations.
BEN was diagnosed using following criteria: (1) farmers living in endangered villages, (2) a familial history positive for BEN, (3) low molecular weight proteinuria, (4) mild proteinuria, (5) impaired kidney function, (6) anemia, and (7) symmetrically shrunken kidneys.Citation12,Citation13 Diagnosis of BEN was established in patients who, in addition to the first two criteria, had either low molecular weight proteinuria or proteinuria and at least one of the remaining criteria but after exclusion of other kidney diseases. Anemia was defined as hemoglobin below 130 g/L for males and postmenopausal women and below 120 g/L for premenopausal women, as proposed by the World Health Organization.Citation14 Impairment of kidney function was defined as glomerular filtration rate estimated using MDRD equation (eGFR) lower than 60 mL/min/1.73 m2.Citation15
GN was diagnosed using routine methods including kidney biopsy. Patients with nephrotic proteinuria were excluded from this study. The histopathological diagnoses of patients with GN were membranous nephropathy in six, IgA nephritis in eight, non-IgA mesangioproliferative GN in six and mesangiocapillary GN in four, focal segmental glomerulosclerosis in 6, and lupus nephritis class II in six patients. A diagnosis of NS was made in patients with hypertension for more than 10 years, a positive family history for hypertension, the presence of hypertensive retinopathy and left ventricle hypertrophy, as well as proteinuria that appeared after hypertension had been detected. Exclusion criteria for this group were renovascular hypertension and other definite causes of hypertension including primary and secondary renal diseases. Two healthy control groups were involved in this study: the first consisted of clinically healthy members of BEN families and the second consisted of healthy persons with negative family history for BEN. Persons invited for control groups were included in this study if no pathological finding was detected by objective examination and routine laboratory examination that comprised urine dipstick test, measurement of serum urea and creatinine levels, calculation of eGFR, and kidney ultrasound.
The Ethics Committee of the Medical Faculty, University of Niš, approved this study, and both patients and healthy controls gave their informed consent.
Laboratory analyses, including peripheral blood cell count and serum levels of urea and creatinine, were performed using standard biochemical methods. At the time of patient inclusion in this study, fresh morning random urine was sampled and centrifuged at 12,000 ×g for 5 min. Supernatant was divided into two samples and in one of them, used for the measurement of beta2-MG, pH was checked. If pH was lower than 7, this portion of urine was alkalized with 1 mol sodium hydroxide until a pH ≥7 and <8 was achieved. All samples were stored at –20°C until assayed. Urine protein was measured using a colorimetric method with pyrogallol red and expressed as mg protein/mmol creatinine (normal value <20°mg/mmol creatinine) and urine albumin using a photometric color method with bromocresol green on analyzer Olympus AU 400 (Olympus Co. Ltd., Tokyo, Japan) (normal value <2.5°mg/mmol creatinine in a male or <3.5°mg/mmol creatinine in a female). Urine alpha1-MG was measured using an immunonephelometric assay (BN II nephelometer, Dade Behring Marburg, Germany) (normal value <1.5°mg/mmol creatinine) and beta2-MG was assayed using the method based on the microparticle enzyme immunoassay technology (Axsym, Abbott Lisnamuck, Ireland) (normal value <33°µg/mmol creatinine).
Statistical Analysis
Descriptive statistics are reported as frequency for categorical data and for continuous data as mean and standard deviation or median and interquartile range as indicated in the legend to the tables. Comparison of the variables among the five groups was made with one-way analysis of variance. Mutual differences between evaluated groups were additionally analyzed using Tukey HSD (in cases where Levene test was not significant) and using Games–Howell (in cases where Levene test was significant) post hoc tests.Citation16 The statistical significance of the differences between the group frequencies was determined using the chi-square test. The correlation between variables was tested using Pearson's correlation coefficient. A value of p°<°0.05 was considered significant. Binary logistic regression analysis was used to find out predictors in differentiating BEN from other kidney diseases (GN and NS group together) and healthy controls.
The clinical accuracy of the examined parameters was assessed using receiver operating characteristic (ROC) curve analysis. ROC plots were constructed and the areas under the curves (AUCs), standard errors, 95% confidence interval, sensitivity, and specificity were calculated using MedCalc computer program. Cutoff values at which the discrimination between the cases with positive diagnosis and those with negative diagnosis was optimal were set. Comparisons of the areas under different ROC plots were made using univariate z-scores.
Statistical analysis was performed using the SPSS software version 16.0 for Windows.
RESULTS
Characteristics of the five examined groups are presented in . Significant difference in gender and age was found between the groups. Group of patients with GN was significantly younger (p < 0.01; post hoc Tukey HSD test) than the other groups and had eGFR similar to healthy controls but significantly higher than two other patient groups. Significant difference among the examined groups was also found in hemoglobin, serum urea and creatinine levels, GFR, and diastolic blood pressure but not in systolic blood pressure. Although none from the control groups referred history of hypertension, blood pressure above 140/90 mmHg was found in 20 persons from non-BEN families and in 13 persons from BEN families. All of them were advised to check their blood pressure with their family doctors.
Table 1. Characteristics of examined groups
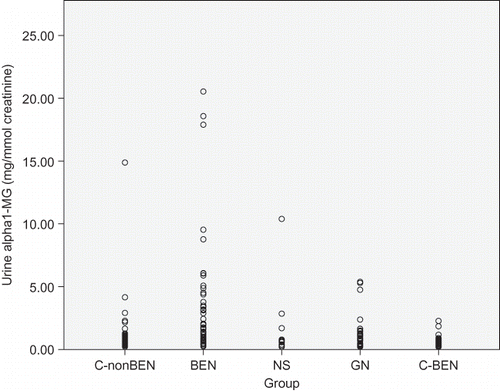
Comparison of urinary marker levels is presented in . Median values of all four markers in patients with NS were similar to those found in healthy persons from both control groups. However, significantly different median values of examined urinary markers were found in BEN and GN groups: the highest proteinuria and albuminuria were found in patients with GN, but the highest urinary excretion of beta2-MG and alpha1-MG was found in BEN patients. Interquartile ranges presented in as well as individual values presented in showed important differences in the dispersion of individual values in the four examined groups. Only few healthy persons and similarly only few patients with NS had urine albumin, beta2-MG, and alpha1-MG above the normal range. Microalbuminuria was frequently found both in patients with GN and with BEN, but increased beta2-MG and alpha1-MG urine excretion was rarely found in GN and frequently in BEN patients.
Table 2. Urine excretion of protein, albumin, and low molecular weight proteins
Figure 1. Individual values of urine albumin in patients with Balkan endemic nephropathy (BEN), glomerulonephritis (GN), nephrosclerosis (NS), healthy controls from non-BEN families (C-nonBEN), and healthy controls from BEN families (C-BEN).
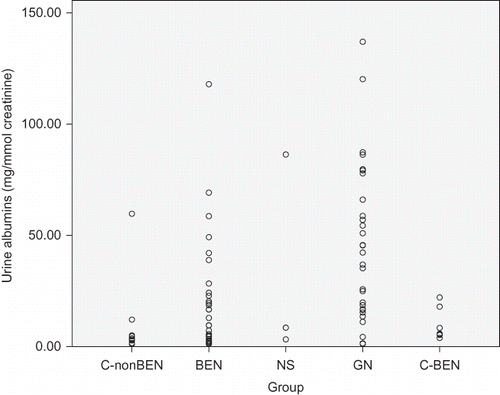
Figure 2. Individual values of urine beta2-microglobulin in patients with Balkan endemic nephropathy (BEN), glomerulonephritis (GN), nephrosclerosis (NS), healthy controls from non-BEN families (C-nonBEN), and healthy controls from BEN families (C-BEN).
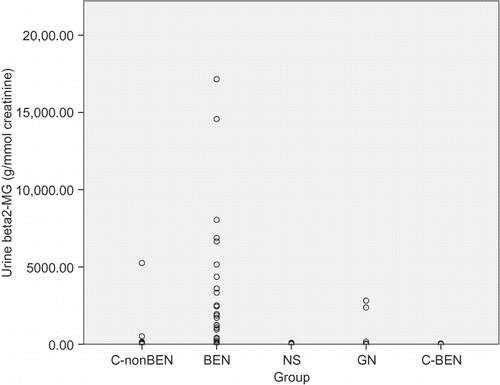
Figure 3. Individual values of urine alpha1-microglobulin in patients with Balkan endemic nephropathy (BEN), glomerulonephritis (GN), nephrosclerosis (NS), healthy controls from non-BEN families (C-nonBEN), and healthy controls from BEN families (C-BEN).
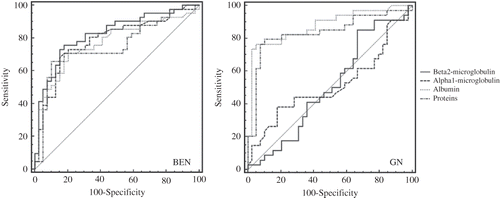
Using the ROC curve analysis, the AUC and 95% confidence interval for examined urinary markers in differentiating patients with BEN, GN, and NS from healthy controls (healthy persons from non-BEN families) were calculated and presented in . In BEN group, the highest AUC was found for urine beta2-MG that was insignificantly different from AUC for alpha1-MG but significantly higher than for urine albumin (, ). In GN patients, AUC for urine protein and albumin was significantly higher than for the two low molecular weight proteins, but in addition AUC for the urine albumin was significantly higher than for the urine protein (, ). In NS group, AUCs for all four urinary markers were significantly lower than in BEN group (p < 0.001).
Table 3. Areas under the curve and 95% confidence interval for four urine markers used in the differentiation of Balkan endemic nephropathy, glomerulonephritis, and nephrosclerosis from healthy controls from nonendemic families
Figure 4. ROC analysis for four examined urinary markers in differentiating BEN from healthy controls (left pannel), and GN from healthy controls (right pannel).
presents the cutoff values corresponding to the best combination of high sensitivity and high specificity of examined urinary markers and their sensitivity and specificity in the differentiation of healthy controls, GN, and NS from BEN. At the cutoff levels, beta2-MG and alpha1-MG had high specificity and satisfactory sensitivity in the differentiation of BEN from healthy controls. In the differentiation of GN from BEN, beta2-MG had high sensitivity, alpha1-MG had high specificity; urinary albumin and protein had high specificity and satisfactory sensitivity. Although in the differentiation of NS from BEN urinary albumin and alpha1-MG had high specificity, their sensitivity is too low to be the useful markers. However, despite the described differences, the values of statistical significance () show that these proteins, at presented cutoff values, can be used as markers for the differentiation of BEN from healthy persons and patients with GN and NS.
Table 4. The results of ROC curves analysis of four urinary markers in healthy persons, patients with glomerulonephritis, and nephrosclerosis in comparison with BEN patients
Univariate/multivariate logistic regression was used to find out predictors in differentiating BEN from healthy controls (healthy persons from non-BEN families) and BEN from other kidney diseases (GN and NS groups together). When age, systolic BP, diastolic BP, hemoglobin, s-urea, GFR (MDRD), and one of the urinary markers (albumin, beta2-MG, alpha1-MG) were combined, only s-urea (β = 0.39; OR = 1.48; p = 0.003) appeared as a significant predictor in the differentiation BEN from healthy controls, and only age (β = –0.29; OR = 0.75; p = 0.000) as a significant predictor in the differentiation of BEN and other kidney diseases. However, collinearity was found among all urinary markers as well as between each of the urinary markers and GFR, and urinary markers and hemoglobin in the BEN group. Therefore, three models were formed involving only noncollinear variables. The models involved systolic and diastolic blood pressures and one of the following urinary markers: beta2-MG, alpha1-MG, and albumin. The analysis revealed albumin (β = 0.06; OR = 1.06; p = 0.019), beta2-MG (β = 0.00; OR = 1.00; p = 0.027), alpha1-MG (β = 0.44; OR = 1.55; p = 0.007) as significant independent predictors for the differentiation of BEN from healthy controls. Albumin (β = 0.03; OR = 1.03; p = 0.009), beta2-MG (β = –0.00; OR = 0.99; p = 0.032), and alpha1-MG (β = –0.26; OR = 0.77; p = 0.029) were also found as significant independent predictors for the differentiation of BEN from other kidney diseases.
DISCUSSION
In this study, the diagnostic value of urinary beta2-MG and alpha1-MG as well as protein and albumin in the differential diagnosis of BEN from healthy persons and patients with GN and NS was examined. As could be expected, the highest urinary excretion of tubular markers, beta2-MG and alpha1-MG, was found in BEN patients, but the highest proteinuria and albuminuria were found in patients with GN. Cutoff values were derived from ROC curves for all four urinary markers that significantly differentiate BEN from healthy persons and patients with GN and NS. Nevertheless, beta2-MG and alpha1-MG had similar sensitivity, specificity, and predictive value in the differentiation of BEN from healthy persons, but urinary protein and albumin are more capable markers for the differentiation of BEN from GN. Although AUCs for all four urinary markers in NS patients were the smallest as compared with those of the two other patient groups, the cutoff values of all these markers could be found that enabled differentiation of BEN from NS.
Beta2-MG (MW 11.7 kDa) and alpha1-MG (MW 27 kDa) are low molecular weight proteins that are freely filtrated through the glomeruli and almost completely reabsorbed by epithelial cells of proximal tubules, where they are metabolized.Citation17,Citation18 Therefore, any proximal tubular cell dysfunction results in increased urinary excretion of low molecular weight proteins. Hall and VasiljevićCitation2,Citation5 were the first to describe increased excretion of beta2-MG in the urine of patients with BEN. After them, a series of authors substantiated increased urinary excretion of beta2-MG in BEN patients and considered it as a sensitive indicator of tubular damage.Citation7,Citation19,Citation20 As beta2-MG is disintegrated in the urine with pH below 6.0, recently alpha1-MG was used for the detection of low molecular weight proteinuria in BEN.Citation12 It has the advantage over beta2-MG because it is stable at a low pH.Citation21 Alpha1-MG has already been shown as a marker of renal tubular dysfunction not only in heavy metal poisoning,Citation22,Citation23 multiple myeloma,Citation24 diabetes,Citation25 but also in BEN.Citation11 As urinary beta2-MG has been widely advocated as a sensitive indicator of tubular proteinuria in BEN and, on the other side, there is no study that compares diagnostic potential of beta2-MG and alpha1-MG, it seems needful to undertake the study to answer the question: which of these two urinary markers is a more reliable marker of BEN? This study has just been undertaken to find the answer to this question. As patients with GN and nonnephrotic proteinuria were used as the main control group, urinary excretion of protein and albumin was examined in addition to urinary excretion of two low molecular weight proteins. The characteristic of each biomarker in differentiating patients with BEN from healthy persons and patients with GN and NS was analyzed using ROC and binary logistic regression analysis. No significant difference was found between AUCs for beta2-MG and alpha1-MG both in the combination of BEN and healthy controls and in the combination of BEN and two other kidney diseases. At the same time, cutoff values could be derived from ROC curves for all four urinary markers that enabled statistically significant differentiation of BEN from healthy controls and patients with GN and NS. Although some differences in sensitivity and specificity of beta2-MG and alpha1-MG at the cutoff level as well as in predictive value of these two urinary markers were found when they were used in the differentiation of BEN from healthy controls and patients with other kidney disease, both these low molecular weight proteins were found to be significant markers for identification of BEN. Therefore, the choice of any of them may be done depending on the opportunity and condition of the study.
Tubular proteinuria is considered as a characteristic of BEN; however, not only tubular but also glomerular and mixed proteinuria were described in BEN patients more than 20 years ago.Citation26–28 In these studies authors underlined high level of low molecular weight proteins in the urine of BEN patients, but they could not neglect the presence of albumin. Karlsson and LenkeiCitation29 were the first who described increased albumin excretion in subjects living in a BEN region. Stefanović and coworkersCitation7 described increased urinary albumin excretion both in BEN patients and in clinically healthy members of BEN families,Citation7 but recently also in children from BEN families.Citation30 In this study, increased urinary excretion of beta2-MG and alpha1-MG was found in 76% and 67% of BEN patients, respectively, but increased urinary albumin excretion in 53% of BEN patients. Hence, although increased excretion of low molecular weight proteins is a frequent finding in BEN patients, albuminuria is also a common finding in BEN. That directed to the already opened question on the localization of lesion in BEN.Citation30,Citation31 Although low molecular weight proteinuria is a marker of tubular damage, increased albumin excretion (MW 68 kDa) indicates an elevated permeability of the glomerular membrane. Mixed glomerular and tubular proteinuria could indicate not only complex but also more advanced kidney damage. Despite numerous papers on proteinuria in BEN patients and in healthy persons from BEN region, further study of characteristics of proteinuria in different stages of the disease is warranted.
The similarity of the morphological and clinical patterns of BEN and Chinese herbs nephropathy (recently named aristolochic acid nephropathy, AAN) has raised the possibility of a common etiologic agent, aristolochic acid, described in 1969 by Ivic and confirmed by recent studies of aristolochic acid–DNA adducts and p53 mutational spectra.Citation32–34
AAN exhibits various profiles of tubular proteinuria that are the hallmarks of the disease.Citation35 This pattern is still detectable in patients with renal failure and/or glomerular albuminuria. In the early-stage AAN, only urinary beta2-MG, retinol-binding protein, and Clara cell protein were increased whereas total proteinuria and Serum creatinine (SCr) were normal. In the later stage, urine albumin, cystatin C, alpha1-MG, and N-acetyl-beta-d-glucosaminidase were also elevated, whereas total proteinuria and SCr were moderately raised. Like in AAN, beta2-MG seems to be an early marker of tubular damage in BEN.
The patients with GN who present the main positive control group consisted of patients with nonnephrotic proteinuria; however, their proteinuria and albuminuria were significantly higher than in BEN patients. In addition, as could be expected, proteinuria and albuminuria were found to have higher diagnostic value in the differential diagnosis of GN from healthy persons than two low molecular proteins, whereas the opposite was found for BEN.
The limitation of this study is only a small group of patients with NS. Our previous study that compared kidney size of patients with BEN, GN, and NS showed that kidney dimensions enabled significant differentiation of BEN patients from GN patients in all stages of the disease. However, there was no difference in the kidney length, width, and depth between patients with BEN and those with NS in stage 1, but the difference appeared in stage 2 of the disease.Citation36 Therefore, we want to check whether the four urinary markers examined in this study could be used as significant markers in the differential diagnosis between BEN and NS. Although a small NS group was examined, the results showed that the cutoff values of all four urinary markers could be used in the differentiation of BEN from NS.
CONCLUSION
Increased urinary excretion of beta2-MG and alpha1-MG was found in 76% and 67% of BEN patients, respectively, but increased urinary albumin and protein excretion was found in one half of BEN patients. Median urine beta2-MG excretion in BEN patients was 17.5 times higher than in GN patients and 18.3 times higher than in controls; median alpha1-MG excretion was higher only 3.0 and 2.25 times, respectively. In the differentiation of BEN from healthy controls, beta2-MG had higher sensitivity and specificity at the cutoff levels (p < 0.001) than alpha1-MG (p < 0.05). In the differentiation of BEN from GN, beta2-MG was the best marker. Like in AAN, beta2-MG seems to be an early marker of tubular damage in BEN.
Nevertheless, all four urinary markers can be used, at identified cutoff values, for the differential diagnosis of BEN, beta2-MG being the best.
ACKNOWLEDGMENTS
This work was supported by grants no. 145004 and no. 175092 from the Ministry of Science and Technological Development of Serbia.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Djukanović L, Marić I, Marinković J, Ignjatović S, Bukvić D. Evaluation of criteria for the diagnosis of Balkan endemic nephropathy. Ren Fail. 2007;29:607–614.
- Hall PW, Gaon J, Griggs PC, The use of electrophoretic analysis of urinary protein excretion to identify early involvement in endemic nephropathy. In: Wolstenholme GEW, Knight J, eds. The Balkan Nephropathy. Ciba Foundation Study Group No. 30. London: Churchill Press; 1967:72–83.
- Bruckner I, Stoica G, Serban M. Studies on urinary proteins. In: Wolstenholme GEW, Knight J, eds. The Balkan Nephropathy. Ciba Foundation Study Group No. 30. London: Churchill Press; 1967:15–20.
- Radošević Z, Traeger J, Radonić M, Manuel Y, Revillard JP. Etude electrophoretique de la proteinurie de 31 sujet crates vivant en pays de nephropathie endemique. J Urol Nephrol. 1968;74:703–710.
- Hall PW, Vasiljević M. Beta2-microglobulin excretion as an index of renal tubular disorders with special reference to endemic Balkan nephropathy. J Lab Clin Med. 1973;81:897–904.
- Stefanović V. Diagnostic criteria for Balkan endemic nephropathy. In: Strahinjic S, Stefanovic V, eds. Current Research in Endemic (Balkan) Nephropathy. Niš: University Press; 1983:351–363.
- Stefanović V, Mitić-Zlatković M, Čukuranović R, Miljković P, Pavlović NM, Vlahović P. B2-microglobulin in patients with Balkan nephropathy and in healthy members of their families. Kidney Int. 1991;40(Suppl. 34):S97–S101.
- Stefanović V, Cukuranovic R, Djordjevic V, Jovanovic I, Lecic N, Rajic M. Tubular marker excretion in children from families with Balkan nephropathy. Pediatr Nephrol. 2009;24:2155–2166.
- Hrabar A, Aleraj B, Čeović S, Čvorišćec D, Vacca C, Hall PW. A fifteen year cohort based evaluation of β2-microglobulin as an early sign of Balkan endemic nephropathy. Kidney Int. 1991;40(Suppl. 34):S41–S43.
- Radovanović Z, Danilović V, Velimirović D, Beta2-microglobulinuria as a predictor of death in a population exposed to Balkan endemic nephropathy. Kidney Int. 1991;40(Suppl. 34):S32–S34.
- Cvoriščec D. Early diagnosis of endemic nephropathy. Clin Chim Acta. 2000;297:85–91.
- Djukanović L, Marinković J, Marić I, Contribution to the definition of diagnostic criteria for Balkan endemic nephropathy. Nephrol Dial Transplant. 2008;23:3932–3938.
- Stefanović V, Jelaković B, Čukuranović R, Diagnostic criteria for Balkan endemic nephropathy: Proposal by an international panel. Ren Fail. 2007;29:867–880.
- Cameron JS. European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 1999;14(Suppl. 2):61–65.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–S266.
- Morgan GA, Leech NL, Gloeckner GW, Barrett KC. SPSS for Introductory Statistics: Use and Interpretation. 2nd ed. Mahwah, NJ: Lawrence Erlbaum; 2004:148–156.
- Ekstrom B, Peterson PA, Berggard I. A urinary and plasma ά1-glycoprotein of low molecular weight: Isolation and some properties. Biochem Biophys Res Commun. 1975;65:1427–1433.
- Karlsson F, Groth T, Sege K, Turnover of human beta2-microglobulin, the constant chain of HLA antigens. Eur J Clin Invest. 1980;10:293–300.
- Radonić M. Beta2-microglobulin in Balkan endemic nephropathy. Pathol Biol. 1978;26:317–320.
- Trnačević S, Halilbašić A, Ferluga D, Renal function, protein excretion and pathology of Balkan endemic nephropathy. I. Renal function. Kidney Int. 1991;40(Suppl. 34):S49–S51.
- Yu H, Yanagisawa Y, Forbes MA, Cooper EH, Crockson RA, MacLennan ICM. Alpha1-microglobulin: An indicator protein for renal tubular function. J Clin Pathol. 1983;36:253–259.
- Chia KS, Tan AL, Chia SE, Ong CN, Jeyaratnam J. Renal tubular function of cadmium exposed workers. Ann Acad Med Singapore. 1992;216:756–759.
- Endo G, Konishi Y, Kiyota A, Horiguchi S. Urinary ά1-microglobulin in lead workers. Bull Environ Contam Toxicol. 1993;50:744–749.
- Honkanen E, Pettersson T, Teppo AM. Urinary ά1- and β2-microglobulin in light chain proteinuria. Clin Nephrol. 1995;44:22–27.
- Hong C-Y, Hughes K, Chia K-S, Ng V, Ling S-L. Urinary ά1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26:338–342.
- Čvoriščec D, Radonić M, Čeović S, Aleraj B. Characteristics of proteinuria in endemic nephropathy. J Clin Chem Clin Biochem. 1983;21:569–571.
- Čvoriščec D, Stavljenić A, Radonić M. Relationship between tubular and Tamm-Horsfall proteinuria in Balkan endemic nephropathy. Nephron. 1986;42:152–155.
- Raičević S, Trnačević S, Hranisavljević J, Vučelić D. Renal function, protein excretion, and pathology of Balkan endemic nephropathy. II. Protein excretion. Kidney Int. 1991;40(Suppl. 34):S52–S56.
- Karlsson FA, Lenkei R. Urinary excretion of albumin and beta2-microglobulin in population from an area where Balkan nephropathy is endemic. Scand J Clin Lab Invest. 1977;37:169–172.
- Stefanovic V, Cukuranovic R, Mitic-Zlatkovic M, Hall PW. Increased urinary albumin excretion in children from families with Balkan nephropathy. Pediatr Nephrol. 2002; 17:913–916.
- Dimitrov P, Tsolova S, Georgieva R, Clinical markers in adult offspring of families with and without Balkan endemic nephropathy. Kidney Int. 2006;69:723–729.
- Cosyns JP, Jadoul M, Squifflet JP, De Plaen JF, Ferluga D, van Ypersele de Strihou C. Chinese herbs nephropathy: A clue to Balkan endemic nephropathy. Kidney Int. 1994;45:1680–1688.
- Ivic M. The problem of etiology of endemic nephropathy. Lijec Vjes. 1969;91:1278–1281.
- Grollman AP, Shibutani S, Moriya M, Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci USA. 2007;104:12129–12134.
- Kabanda A, Jadoul M, Lauwerys R, Bernard A, van Ypersele de Strihou C. Low molecular weight proteinuria in Chinese herbs nephropathy. Kidney Int. 1995;48:1571–1576.
- Ležaić V, Marić I, Jovanović D, Comparison of kidney size between patients with Balkan endemic nephropathy and other kidney diseases. Kidney Blood Press Res. 2008;31:307–312.