Abstract
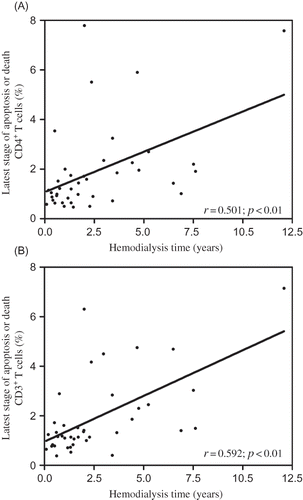
End-stage renal disease (ESRD) under hemodialyses (HD) is related with a higher propensity to infections, essentially due to T-cell lymphopenia. We postulated that HD procedure affects CD4+ T cells, especially by inducing apoptotic death and that recombinant human erythropoietin (rhEPO) therapy may also play an important role in the modulation of the immune system in these patients. T-cell phenotype and apoptosis of HD patients and healthy controls were evaluated by flow cytometry using anticoagulated whole-blood samples. In 12 HD patients, these parameters were also analyzed before and immediately after HD procedure. HD patients showed a decrease in total circulating CD3+ lymphocytes, especially in CD4+ T cells (0.747 ± 0.410 vs. 0.941 ± 0.216 × 109/L, p < 0.05), which could be a consequence of the higher proportion of CD3+ and CD4+ lymphocytes in the latest stage of apoptosis (or death) and of the higher proportion of apoptotic CD4+ T cells observed in the patients immediately after HD procedure (2.91 ± 0.780 vs. 3.90 ± 1.96, p < 0.05). A positive and statistically significant correlation between CD3+ and CD4+ lymphocytes in latest stage of apoptosis (or death) with HD time was found (CD3+: r = 0.592, p < 0.01; CD4+: r = 0.501, p < 0.01). We also found a negative and significant correlation between weekly rhEPO doses and the number of CD4+ T cells (r = –0.358, p < 0.05). In conclusion, HD procedure still contributes to the development of T-cell lymphopenia, at least in part, by apoptosis induction. It was also shown that rhEPO therapy is associated with the CD4+ T-cell decline, possibly by immune modulation, eliminating atypical cells and helping to restore the CD4+ T-cell subset.
INTRODUCTION
In the past few years, many efforts were done to ameliorate and increase life quality and survival of end-stage renal disease (ESRD) patients under hemodialysis (HD). However, ESRD remains a global public health challenge due to its increased prevalence in the population and high mortality and morbidity, mainly associated to cardiovascular disease.Citation1 Moreover, infections are the second most frequent cause of death in HD patients, which are related with an acquired cellular immunodeficiency.Citation1 This is expressed by a high susceptibility to infections and infectious complications, and also by the low rate of response to vaccines,Citation2 increased incidence of tumors,Citation3 and delayed hypersensivity.Citation4
In previous studies performed by our group, a decrease in the number of circulating T cells was found in HD patients, both in CD4+ and in CD8+ T-cell compartments.Citation5,Citation6 There are some possible explanations for T-cell depletion in HD patients, namely, higher turnover, disturbances in lymphocyte homeostasis due to uremia, and increased peripheral lymphocyte apoptosis associated with activation stimulus.7–9 Furthermore, we also reported increased levels of activation in the markers HLA-DR and CD57 in CD4+ and CD8+ T-cell compartments from HD patients.Citation5 In fact, CD57 expression in T cells is recognized as a marker of in vitro replicative senescenceCitation10 and as an indicator of programmed cell death.Citation11–13 In addition, cell death receptors, such as Fas (CD95), have been demonstrated to be upregulated in activated lymphocytes.Citation14 These data may suggest an increased susceptibility of CD4+ and CD8+ T cells to programmed cell death by apoptosis in HD patients.
Erythropoietin (EPO) mainly produced by kidney is the crucial regulator of erythroid lineage. The cloning of its gene led to the introduction of recombinant human erythropoietin (rhEPO) into clinical practice for the treatment of several anemias. However, during the past decade, the therapeutic properties of rhEPO have been thoroughly investigated and as a consequence, clinical applications of rhEPO have been extended, namely, to the improvement of both cellular and humoral immune responses.Citation15,Citation16
We hypothesized that both the HD procedure and rhEPO therapy could be related with the impairment in CD4+ T-cell apoptosis. There are several studiesCitation7,Citation8,Citation17,Citation18 referring disturbances in the lymphocyte populations of ESRD, as compared to controls; however, there are fewCitation19 studies about the effect of the HD procedure, by studying these cells immediately before and after HD. Our aim was to evaluate an earlier marker of apoptosis through Annexin-V assay using flow cytometry, in a group of ESRD patients and controls, as well as in a group of patients before and after the HD procedure. In this way, we are able to evaluate the modifications induced by long-term HD and immediately after the procedure.
MATERIAL AND METHODS
Subjects
We performed a cross-sectional study by evaluating 47 ESRD patients under HD (26 males, 21 females; mean age ± SD: 65.58 ± 16.99 years) and rhEPO therapy. Twelve of these patients were also evaluated before and immediately after HD procedure.
Patients with evidence of acute or chronic infection, malignancy, and hematological disorders were excluded. All patients gave their informed consent to participate in this study.
Healthy volunteers were selected as controls based on normal hematological and biochemical values, and had no history of kidney or inflammatory diseases. They were also matched as far as possible for age and gender with the HD patients.
Assays
Blood samples were collected (EDTA as anticoagulant) from ESRD patients, before starting HD. To evaluate the effect of HD in the studied parameters, blood samples were also collected immediately after HD.
The hemoglobin concentration and white blood cell count were measured using an automatic blood cell counter (Sysmex K1000, Hamburg, Germany) and leukocyte differential counts were evaluated in Wright-stained blood films.
Flow Cytometry Analysis
For the immunofluorescence cell staining, 200 µL of each blood sample was subjected to a whole-blood stain-lyse-and-wash method, using the florescence-activated cell sorting (FACS) lysing solution [Becton-Dickinson Biosciences (BDB), San Jose, CA, USA]. Cells were incubated with fluorescein isothiocyanate anti-human CD3 or CD4 (Biolegend, San Diego, CA, USA) in phosphate-buffered saline (PBS) containing 3% FCS for 30 min. The cells were washed twice with phosphate-buffered saline–3% fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA) and resuspended in 100 µL binding buffer (10 mM HEPES buffer containing 0.14 mM NaCl and 2.5 mM CaCl2, pH 7.4) containing 5 µL of phycoerythrin-conjugated Annexin-V (Pharmingen, San Diego, CA, USA), and 5 µL of 7-amino-actinomycin (7-AAD) (Pharmingen), and incubated for 15 min at room temperature in the dark. The stained cell suspensions were then diluted in binding buffer to a final volume of 0.5 mL and immediately analyzed. As a positive control of apoptosis, we used cells that were incubated for 12 h with 1 µM staurosporine (Sigma-Aldrich). Flow cytometric analysis was carried out in a florescence-activated cell sorting calibur (San Jose, CA, USA) and was based on the acquisition of 20,000 events. Detectors for forward and side light scatter were set on a linear scale, whereas logarithmic detectors were used for all three fluorescence channels (FL-1, FL-2, and FL-3). Compensation for spectral overlap between FL channels was performed for each experiment using single-color-stained cell populations of the positive control. All data were collected ungated to disk and were analyzed using CELLQuest Pro software. Lymphocytes were gated on the basis of their forward and side-scatter characteristics and then on CD4 or CD3 expression and the numbers of such lymphocytes were determined afterward. CD3- or CD4-positive lymphocytes were then analyzed for their expression of Annexin-V and 7-AAD, and this frequency was applied to the total number of CD3+ and CD4+ T cells to determine the number of viable cells the Annexin-V and 7-AAD negative (Annexin-V−/7-AAD−) cells; the number of cells undergoing apoptosis, the Annexin-V positive and 7-AAD negative (Annexin-V+/7-AAD−) cells; and the number of dead cells or cells that were in the latest stage of apoptosis, Annexin and 7-AAD positive (Annexin-V+/7-AAD+) cells.
Data Analysis
For statistical analysis, we used the Statistical Package for Social Science, version 17.0. Kolmogorov–Smirnov test was used to evaluate normality distribution, and the parameters are expressed as mean ± SD or as median values (inter-quartile range) when appropriate. Single comparisons were performed with the Student's t-test whenever the parameters presented a Gaussian distribution and the Mann–Whitney U-test in the case of a non-Gaussian distribution. To compare data before and after HD, we used paired-samples t-test or Wilcoxon test. Spearman's rank correlation coefficient was used to evaluate relationships between sets of data. Significance was accepted at p < 0.05.
RESULTS
HD patients were dialyzed three times per week for 3–5 h, for a median time of 1.9 years. All patients used the high-flux polysulfone FX-class dialyzers of Fresenius (Fresenius Medical Care, Bad Homburg, Germany). The mean Kt/V value was 1.43 ± 0.29, which reflects the adequacy of the HD process in our patients. The causes of renal failure in these patients were diabetic nephropathy in 22, hypertensive nephrosclerosis in 8, polycystic kidney syndrome in 4, pyelonephritis associated with neurogenic bladder in 1, chronic interstitial nephritis in 2, and uncertain etiology in 10 patients. The vascular access for HD was arteriovenous fistula in 37 patients and central venous catheter in 10 patients. The HD patients included were under rhEPO treatment, which is administrated weekly, with a median dose of 0.32 µg/kg, in an attempt to achieve the target hemoglobin levels, 11–12 g/dL.
The hematological characteristics of HD patients and controls are summarized in .
Table 1. Blood cell count and T-cell phenotype in controls and HD patients
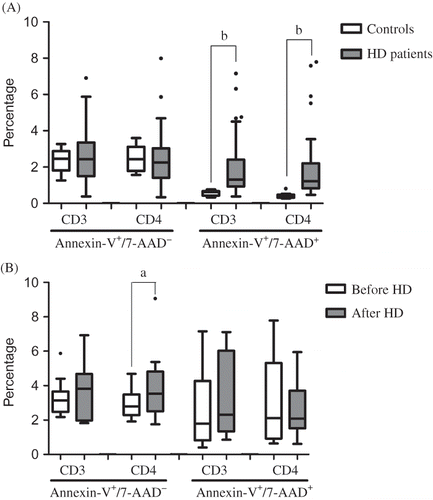
In this study, we found a significant increase in monocytes and a trend for lower lymphocyte counts in HD patients, as compared to controls. No statistically significant differences were found in total white blood cells, neutrophil, and eosinophil counts, and in neutrophil/lymphocyte ratio, between controls and HD patients. However, HD patients showed a significant decrease in total circulating CD3+ lymphocytes, essentially in CD4+ T-cell compartments ().
HD patients showed a statistically significant decrease in the proportion of CD3+ and CD4+ viable lymphocytes, associated with significant increase in the percentage of CD3+ and CD4+ lymphocytes in the latest stage of apoptosis or death. However, no statistically significant differences were found between controls and HD patients concerning the percentage of apoptotic T cells ().
Figure 1. Apptosis evaluation by Annexin-V assay, in a group of patients and controls (A) and in a group of patients before and after the HD procedure (B). Two different CD3+/CD4+ T-cells populations were classified according to the differents staining of Annexin-V and 7-AAD: cells underdoing apoptosis (Annexin-V+/7-AAD−) and cells in the latest apoptosis stage or death (Annexin-V+/7-AAD+). a) P < 0.05; b) p < 0.01; HD = hemodialyse
A positive and statistically significant correlation was found between CD3+ and CD4+ lymphocytes in the latest stage of apoptosis or death with the time that patients were under HD therapy ( and B). We also found a negative and significant correlation between weekly rhEPO doses and the number of CD4+ T cells (r = –0.358; p < 0.01). Additionally, when HD patients were divided according to the median time under HD therapy (2 years), we found that patients with more than 2 years had a significant decrease in the lymphocyte count (1.48 ± 0.56 vs. 1.98 ± 0.97 × 10Citation9/L, p < 0.05).
Figure 2. Correlations between Hemodialysis time with the proportion of (A) CD4+ and (B) CD3V+ T cells that died or are in the latest stage of apoptosis.
Analyzing the results for lymphocyte counts, T-cell phenotype, and apoptotic CD4+ T cells, before and immediately after the HD procedure ( and ), we found a significantly higher proportion of apoptotic CD4+ T cells.
Table 2. Lymphocyte count and T-cell phenotype in HD patients, before and immediately after HD procedure
DISCUSSION
Several immunological abnormalities have been reported in ESRD patients, including a low number of circulating T, B, and NK lymphocytes,Citation7 unspecific activation and reduced production of antibodies by B lymphocytes, defective antigen presentation, B7-2 dependence by monocytes,Citation9 decreased neutrophils phagocytic function,Citation20 and aberrant T-cell-mediated immunity.Citation7,Citation10
Some authors have associated uremia with T-cell inflammatory response inability, as these lymphocytes are easily stimulated in vitro and dialyses may trigger the immune response in these patients.Citation9 Actually, it was reported that phenotypically T cells from patients with ESRD are in an activated state and display altered proliferative responses after stimulation with mitogens or soluble antigens in vitro.Citation7 In addition, our groupCitation5,Citation6 and others,Citation7,Citation8,Citation19 have reported that HD patients have a significant decrease in T-lymphocytes. A possible explanation for this feature is the increase of apoptosis in these cells. In fact, our results show a significant higher proportion of T cells in latest stage of apoptosis or death, namely CD4+ T cells, in HD patients, as compared to healthy controls (). Thus, to clarify whether the HD process itself was responsible for the increase of CD4+ apoptosis, we evaluated Annexin-V binding to CD4+ T-lymphocytes, as an earlier marker of cell death by apoptosis, before and immediately after HD procedure. We found a higher apoptotic cell proportion in post-HD patients, showing the contribution of the HD procedure in the induction of the programmed cell death (). These findings suggest that the contact of the peripheral blood cells with the dialyzer membrane can induce apoptosis of peripheral blood mononuclear cells as described by others.Citation21
Recent data of our group reported a significant increase in the proportion of CD4+ CD57+ T cells in HD patientsCitation5 after the HD procedure. These cells are characterized by their inability to undergo new cell division cycles and associated with a high rate of spontaneous and activation-induced apoptotic death.Citation22 Additionally, CD57+ T-lymphocytes have increased induction of pro-apoptotic molecules, as the caspase-3 and Fas/FasL, and a decreased expression of anti-apoptotic molecules, as survivin.Citation10
These findings support our hypothesis and results. During the HD process peripheral blood lymphocytes contact dialyzer membrane getting an activated state, for example, as CD57-stimulated T cells, marking them to a later and probable apoptotic death, explaining the higher proportion of apoptotic CD4+ T cells in post-HD patients. The long-term HD procedure, with the continuous and repeated T cells HD-induced activation, may be responsible for the higher proportion of CD4+ T cells in the latest stage of apoptosis or dead CD4+ T cells, explaining the increased proportion of these cells in HD patients. In support of this, our results show that the HD patients with time of HD therapy above the HD median time present a significant decrease in the lymphocyte count when compared to the patients with lower HD time. In addition, significant and positive correlations between T cells in the latest stage of apoptosis or death with HD time were observed ( and B).
Interestingly, we also found a negative and statistically significant correlation between weekly rhEPO dose and CD4+ lymphocyte count, which might be related with inflammation and/or rhEPO immunomodulation.
Higher doses of rhEPO required by some HD patients to achieve the target hemoglobin levels are associated with an enhanced inflammation process, which has been associated with the decrease in CD4+ T-cell count.Citation5 On the other hand, it has been described that long-term rhEPO therapy in HD patients is associated with a decrease of terminally differentiated CD8+ T-cells due to apoptotic death.Citation23 However, the statistically significant correlation between rhEPO doses and CD4+ lymphocyte count that we found needs to be interpreted with some caution as there are potential confounders (parathyroid hormone, iron store, dialysis duration, and others), and further studies are needed to evaluate this correlation correctly.
In conclusion, our data suggest that despite the technological advances, HD procedures still contribute to the development of T-cell lymphopenia due to, at least in part, the induction of cell death by apoptosis. Our results also showed that rhEPO therapy plays an important role in the CD4+ T cells decrease, possibly by an immunomodulatory mechanism, eliminating aberrant cells, and helping to rebuild the CD4+ T-cell compartment. However, further studies are needed to elucidate how rhEPO can affect T cells in these patients and the mechanisms responsible for CD4+ T-cell apoptosis. Finally, it would also be interesting to evaluate the effects of HD procedure and rhEPO therapy in CD8+ T-cell compartment and to evaluate the link and impact of CD57 in these events.
Acknowledgments
This work was supported by the “Fundação Portuguesa para a Ciência e Tecnologia” Project PIC/IC/83221/2007.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- US Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2009.
- Krishnamurthy G, Kher V, Naik S. Low response to HBsAg vaccine in chronic renal failure patients is not due to intrinsic defect of B cells. Scand J Urol Nephrol. 2002;36:377–382.
- Maisonneuve P, Agodoa L, Gellert R, Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet. 1999;354:93–99.
- Selroos O, Pasternack A, Virolainen M. Skin test sensitivity and antigen-induced lymphocyte transformation in uraemia. Clin Exp Immunol. 1973;14:365–370.
- Costa E, Lima M, Alves JM, Inflammation, T-cell phenotype, and inflammatory cytokines in chronic kidney disease patients under hemodialysis and its relationship to resistance to recombinant human erythropoietin therapy. J Clin Immunol. 2008;28:268–275.
- Costa E, Rocha S, Rocha-Pereira P, Neutrophil activation and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Am J Nephrol. 2008;28:935–940.
- Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204–212.
- Litjens N, Druningen CJ, Betjes MGH. Progressive loss of renal function is associated with activation and depletion of naïve T lymphocytes. Clin Immunol. 2006;118:83–91.
- Girndt M, Sester M, Sester U, Kaul H, Köhler H. Molecular aspects of T and B cell function in uremia. Kidney Int. 2001;59:206–211.
- Brenchley JM, Karandikar NJ, Betts MR, Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720.
- Wood KL, Twigg HL, III, Doseff AI. Dysregulation of CD8+ lymphocyte apoptosis, chronic disease, and immune regulation. Front Biosci. 2009;14:3771–3781.
- Wood KL, Knox KS, Wang Y, Day RB, Schnizlein-Bick C, Twigg HL, III. Apoptosis of CD57+ and CD57− lymphocytes in the lung and blood of HIV-infected subjects. J Clin Immunol. 2005;117:294–301.
- Shinomiya N, Koike Y, Koyama H, Analysis of the susceptibility of CD 57+ T cells to CD3-mediated apoptosis. Clin Exp Immunol. 2004;139:268–278.
- Calopa M, Bas J, Callén A, Mestre M. Apoptosis of peripheral blood lymphocytes in Parkinson patients. Neurobiol Dis. 2010;38:1–7.
- Katz O, Lindor G, Lifshitz L, Erythropoietin enhances immune responses in mice. Eur J Immunol. 2007;37:1584–1593.
- Prutchi-Sagiv S, Neumann D, Mittelman M. Erythropoietin as an Immunotherapeutic agent: New uses for an old drug? Med Hypotheses Res. 2005;2:587–596.
- Ueki Y, Nagata M, Miyake S, Tominaga Y. Lymphocyte subsets in hemodialysis patients treated with recombinant human erythropoietin. J Clin Immunol. 1993;4:179–287.
- Kurz P, Köhler H, Meuer S, Hütteroth T, Meyer Zum Büshenfeld KH. Impaired cellular immune responses in chronic renal failure: Evidence for a T cell defect. Kidney Int. 1986;29:1209–1214.
- Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70:371–376.
- Sardenberg C, Suassuna P, Andreoli MCC, Effects of uraemia and dialysis modality on polymorphonuclear cell apoptosis and function. Nephrol Dial Transplant. 2006;21:160–165.
- Carracedo J, Ramírez R, Soriano S, Martín-Malo A, Rodríguez M, Aljama P. Caspase-3-dependent pathway mediates apoptosis of human mononuclear cells induced by cellulosic hemodialysis membranes. Nephrol Dial Transplant. 2002;17:1971–1977.
- Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–8423.
- Trzonkowski P, Debska-Slizien A, Szmit E, Long-term therapy with recombinant human erythropoietin increases CD8+ T-cell apoptosis in hemodialysis patients. Nephrol Dial Transplant. 2005;20:367–376.