Abstract
Objective: This study aims to investigate the epidemiology, clinical and histological features, and prognosis of acute kidney injury (AKI) according to RIFLE classification in adult patients with idiopathic nephrotic syndrome. Methods: In this retrospective study, 277 patients with idiopathic nephrotic syndrome were reviewed from June 2005 to June 2009. Results: Fifty-one (18%) patients entered RIFLE class Risk (AKI-R); 24 (9%) patients entered RIFLE class Injury (AKI-I); and 20 (7%) patients entered RIFLE class Failure (AKI-F). Logistic regression analysis showed that severe hypoalbuminemia, increase in age, and being male were risk factors of AKI. Cumulative recovery rates in 3 months for groups AKI-R, AKI-I, and AKI-F were 95%, 100%, and 94%, respectively (p = 0.21). The mean time to recovery for groups AKI-R, AKI-I, and AKI-F was 20 ± 3, 25 ± 4, and 30 ± 5 days, respectively. Cumulative complete remission rates in 3 months for groups AKI-R, AKI-I, and AKI-F were 92%, 86%, and 65%, respectively (p = 0.002). The mean time to remission for groups AKI-R, AKI-I, and AKI-F was 28 ± 3, 39 ± 6, and 62 ± 8 days, respectively. Conclusion: AKI is not uncommon in adult idiopathic nephrotic syndrome. More severe AKI was associated with longer time of nephrotic syndrome complete remission. Renal function can recover completely in most of the patients.
INTRODUCTION
Acute renal failure (ARF) has been observed in patients with idiopathic nephrotic syndrome.Citation1–9 In the last century, there were reports that approximately 30% of children and adults with idiopathic nephrotic syndrome had significant decrease in glomerular filtration rate (GFR).Citation3,Citation4 There are no studies to report acute kidney injury (AKI) according to Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease (RIFLE) classification in patients with idiopathic nephrotic syndrome since the establishment of new AKI definition and classification by Acute Dialysis Quality Initiative in 2004.Citation8 This study aims to examine the epidemiology, clinical and histological features, and prognosis of AKI according to RIFLE classification in these patients and evaluate factors that may affect the GFR reduction and prognosis of these patients.
SUBJECTS AND METHODS
Participants
For this retrospective study, 277 patients with idiopathic nephrotic syndrome were reviewed from June 2005 to June 2009. The entry criteria were as follows: patients fit the diagnosis of idiopathic nephrotic syndrome, had follow-up for at least 3 months after admission, and were older than 16 years. Patients with lupus nephritis, diabetic nephropathy, purpura nephritis, hepatitis-associated nephritis, acute glomerular nephritis, acute progressive glomerular nephritis, and secondary nephrotic syndrome were excluded. Patients were classified according to the maximum RIFLE class (class R, class I, or class F).
Classification of AKI
The change of serum creatinine level was used to classify patients according to the RIFLE criteria. Patients who met any of the criteria of the RIFLE classification were classified as AKI patients. The mean creatinine value of patients without AKI or the CrMDRD creatinine was used as the baseline value.
Definition
A nephrotic syndrome was defined as the presence of edema, proteinuria in excess of 3.5 g/day, and serum albumin less than 30 g/L. Renal recovery was defined as the return of decreased kidney function to pre-AKI baseline levels. Acute tubular injury on renal biopsy was defined by the presence of tubular simplification, loss of brush border, and enlarged reparative nuclei with nucleoli, with or without mitotic figures. Complete remission was defined as urine protein excretion of <0.5 g/day.
Data Collection
Standard demographic, clinical, and pathologic changes, and laboratory data were retrieved. Demographic information included age and sex. Clinical data included blood pressure (BP) and diagnosis and need for renal replacement therapy. Pathologic data included microscopic and immunofluorescent description. Laboratory data included serum creatinine, serum albumin, hemoglobin, hematocrit, and 24 h proteinuria levels. Serum creatinine was used to calculate the GFR assessed by the Modification of Diet in Renal Disease (MDRD) equation.
Study End Point and Follow-Up
The primary study end point was renal function recovery. The secondary end point was complete remission of nephrotic syndrome. Patients would have follow-up for at least 3 months after admission. Serum creatinine was measured weekly in the hospital. After patients improved clinically and were discharged from hospital, they were followed up in outpatient service and biochemical investigation (including serum creatinine and serum albumin measurement) and urine protein measurement were done biweekly or monthly according to patients' clinical situation.
Statistical Methods
Normally or near normally distributed variables are reported as means with standard deviation (SD) and compared by Student's t-test or analysis of variance test. Categorical data were tested using the chi-square test. Multivariable logistic regression analysis was used to assess the association of AKI with risk factors. Kaplan–Meier time-to-event curves were generated for AKI patients and compared using the log-rank test. Relative incidence rate estimates for end points were calculated using multivariable Cox proportional hazards regression analysis. All statistical tests were two-tailed. p ≤ 0.05 was considered statistically significant. Data are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Data were analyzed using the SPSS version 16 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Incidence of AKI in Patients with Idiopathic Nephrotic Syndrome
A total of 277 patients with idiopathic nephrotic syndrome were enrolled. Of them 182 (66%) patients were non-AKI, 51 (18%) patients entered RIFLE class Risk (AKI-R), 24 (9%) patients entered RIFLE class Injury (AKI-I), and 20 (7%) patients entered RIFLE class Failure (AKI-F). No hypotension, renal vein thrombosis, drug-induced interstitial nephritis, and urinary obstruction were found in these AKI patients.
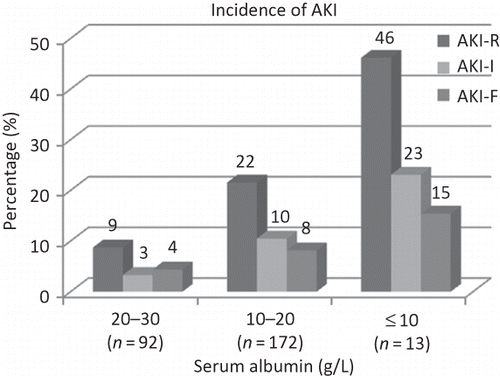
Incidence of AKI According to Patients’ Serum Albumin Level
There were 92 patients with serum albumin level at 20–30 (g/L), and 15 (16%) of these had AKI. There were 172 patients with serum albumin level at 10–20 (g/L), and 69 (40%) of these had AKI. There were 13 patients with serum albumin level no more than 10 (g/L), and 11 (85%) of these had AKI. Incidences of each AKI category in patients with different serum albumin levels are shown in . Logistic regression analysis showed that severe hypoalbuminemia, older age, and being males were risk factors for incidence of AKI ().
Table 1. Logistic regression analysis for incidence of AKI
Baseline Clinical and Serologic Characteristics of Idiopathic Nephrotic Syndrome Patients
There were no significant differences in age, BP, serum sodium, and urine specific gravity among the groups at baseline. Serum albumin in AKI patients were significantly lower compared with non-AKI patients. Compared with the other three groups, hemoglobin was significantly lower and 24-h proteinuria was significantly higher in patients with AKI-F ().
Table 2. Baseline clinical and serologic characteristics of idiopathic nephrotic syndrome patients
Pathological Diagnosis of AKI Patients Who Had Renal Biopsy
Renal biopsies were not performed in most of these AKI patients. Only 32 of 95 AKI patients had renal biopsy. Sixteen had minimal change disease (MCD). One had mild mesangial proliferative glomerular disease. Five had IgA nephropathy and mild mesangial proliferative glomerular disease in microscopic description. Of 32 patients, 20 had renal biopsies during an episode of AKI. There was little interstitial cellular infiltration and no obvious interstitial edema in these patients. AKI classification and acute tubular injury histological change of these 20 patients are shown in .
Table 3. Acute tubular injury in patients during episode of AKI
Treatment of Idiopathic Nephrotic Syndrome Patients with AKI
Thirteen patients refused to receive corticosteroids and cytotoxic drugs. The majority (82/95) of patients received oral corticosteroids as initial treatment. Second-line agents including cyclophosphamide, mycophenolate mofetil, Tripterygii wilfordii, and leflunomide were prescribed for indications that included steroid resistance or dependence, partial response to steroids, and toxicity or contraindications to steroids. Some received more than one agent (usually sequentially) when they failed to respond to or experienced adverse effects from other agents. Only seven AKI-F patients received renal replacement therapy. The treatment line of each AKI group is shown in . Six patients required temporary hemodialysis that ranged from 2 to 10 weeks and one patient needed long-term hemodialysis because of progressing into end stage of renal disease ().
Table 4. Treatment of each AKI group
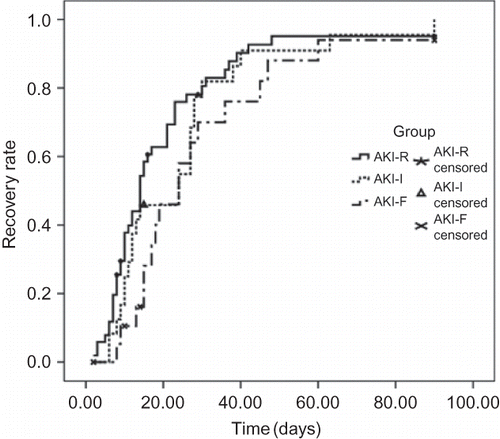
Outcome of Renal Function
There was no significant difference of renal function recovery rate among the three groups. Cumulative recovery rates in 3 months for groups AKI-R, AKI-I, and AKI-F were 95%, 100%, and 94%, respectively. The mean time to recovery for groups AKI-R, AKI-I, and AKI-F was 20 ± 3, 25 ± 4, and 30 ± 5 days, respectively (p = 0.21, overall; p = 0.45, AKI-R vs. AKI-I; p = 0.08, AKI-R vs. AKI-F; p = 0.78, AKI-I vs. AKI-F) (). Cox regression analysis found the incidence of renal function recovery for immunosuppressive therapy compared with no immunosuppressive therapy is 3.4 (CI 1.19–9.70, p = 0.02).
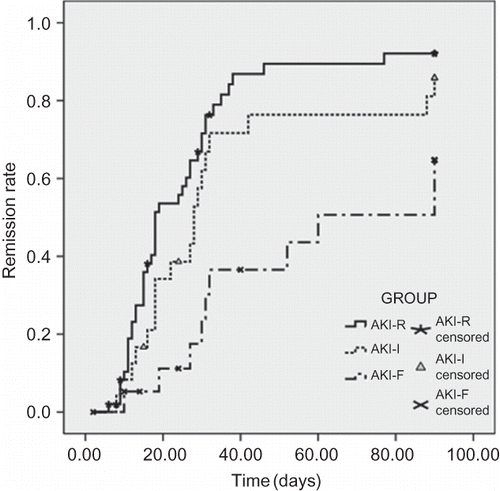
Outcome of Remission
Cumulative complete remission rates in 3 months for groups AKI-R, AKI-I, and AKI-F were 92%, 86%, and 65%, respectively. The mean time to remission for groups AKI-R, AKI-I, and AKI-F was 28 ± 3, 39 ± 6, and 62 ± 8 days, respectively (p = 0.002, overall; p = 0.17, AKI-R vs. AKI-I; p < 0.001, AKI-R vs. AKI-F; p = 0.03, AKI-I vs. AKI-F) (). Cox regression analysis found the incidence of complete remission of nephrotic syndrome for immunosuppressive therapy compared with no immunosuppressive therapy is 9.2 (CI 1.24–68.3, p = 0.03).
DISCUSSION
This study is one of the largest single-center studies to investigate the epidemiology, clinical and histological features, and prognosis of AKI according to RIFLE classification in adult idiopathic nephrotic syndrome patients. There were 34% of idiopathic nephrotic syndrome patients that had AKI according to RIFLE classification in this study. Similar findings have been reported in other series of adults with MCD.Citation3,Citation4
Smith and HayslettCitation10 reviewed the literature from 1966 to 1992 and found risk factors of ARF in nephrotic syndrome patients to be older age (~60 years of age), hypertension, heavy proteinuria, severe lower serum albumin, and being male. In our study, logistic regression analysis also showed that older age, being male, or lower serum albumin patients had higher incidence of AKI. The severe hypoalbuminemia was associated with higher incidence of AKI in idiopathic nephrotic syndrome patients, but there was no significant difference of serum albumin among AKI-R, AKI-I, and AKI-F.
In this study, renal biopsy was not performed in most of these AKI patients. Only one-third of patients had renal biopsy and most of the patients had minimal lesions. Renal biopsies were performed in 20 patients during an episode of AKI and acute tubular injury was found in 11 of them. Three of 11 AKI-R patients and 2 of 3 AKI-I patients and all of 5 AKI-F patients had acute tubular injury change in the histology. Jennette and FalkCitation5 had reported similar findings that tubular epithelial simplification identical to that observed with ischemic ARF (acute tubular necrosis) was observed in 71% of the patients with serum creatinine greater than 177 µmol/L (greater than 2.0 mg/dL) and 0% of those with less than 133 µmol/L (less than 1.5 mg/dL). Other reports had also shown that 60% AKI patients with adult MCD nephrotic syndrome had tubulointerstitial changes compatible with acute tubular necrosis.Citation10,Citation11
Successive increases in severity of RIFLE category were associated with higher mortality in critically ill patients and the RIFLE classification was useful for predicting full recovery of renal function.Citation12–17 In this study, there was no significant difference of recovery rate and the mean time to recovery among AKI-R, AKI-I, and AKI-F groups, although there was a tendency that it took longer time to recovery in more severe AKI patients. Further analysis showed that renal function recovery was affected mainly by immunosuppressive therapies. Other reports also had shown prednisolone therapy improved the renal function.Citation6,Citation18–22 In the majority of reports, the prognosis for recovery of renal function had been good even in patients in whom dialysis was required. This study confirms renal function can recover completely in most of the patients.
In this study, more severe AKI was associated with lower complete remission rate and longer time to remission in idiopathic nephrotic syndrome patients. This finding has not been documented in other literatures. Because more AKI-F patients refused to use immunosuppressive agents than AKI-R and AKI-I patients, the difference of remission rate and time to remission among three groups may be due to different treatment protocols of immunosuppressive agents. Cox regression analysis found the severity of AKI was one of the important factors that affected the prognosis of complete remission except immunosuppressive therapy and age.
Causes for AKI in the nephrotic syndrome patients include rapid original glomerular disease, hypovolemia, renal vein thrombosis, interstitial nephritis, and administration of drugs (radiocontrast, diuretics, and cyclooxygenase inhibitors).Citation20,Citation23,Citation24 Such factors could not be identified in our AKI cases. AKI in idiopathic nephrotic syndrome patients may be defined as idiopathic ARF, which was described in detail by Chamberlain et al.Citation25 as a condition that complicates pre-existing idiopathic nephrotic syndrome with the histological picture of normal or near-normal glomeruli. It is difficult to differentiate idiopathic ARF from prerenal ARF. The predominant role of hypovolemia in the development of nephrotic edema has been questioned and the majority of patients with nephrotic syndrome do not experience hypovolemia.Citation26–29 However, Furuya et al.Citation6 had reported that four nephrotic syndrome patients with ARF had moderate to severe decrease of the effective renal plasma flow measured by p-aminohippurate clearance in condition of no hypovolemia. In the report of ARF by Vande Walle,Citation21 on comparing children with minimal change nephrotic syndrome with patients with minimal change nephropathy disease in remission, AKI patients had lower effective renal plasma flow. From the relationship of AKI category with serum albumin and acute tubular injury in our study, we also think the decrease of effective renal plasma flow is one of the important factors of AKI in adult idiopathic nephrotic syndrome patients, although several other factors may contribute to the decline of acute glomerular filtration in idiopathic nephrotic syndrome, such as high intratubular pressure, low filtration fraction caused by impaired glomerular permeability, tubular obstruction by protein casts, and interstitial edema of the kidney.Citation6,Citation10,Citation11,Citation19–22
We recognize several limitations of this study. As this is a retrospective and single-center study, it may not be representative of all adult idiopathic nephrotic syndrome patients with AKI. Also, only one-fifth of patients underwent renal biopsies during AKI, so the relationship of AKI severity and histological changes cannot represent all patients.
In summary, AKI is not uncommon in adult idiopathic nephrotic syndrome, especially in older and severe hypoalbuminemia patients. More severe AKI was associated with longer time of nephrotic syndrome complete remission. Renal function can recover completely in most of the patients. Immunosuppressive therapies to induce nephrotic syndrome remission may play a very important role in renal function recovery.
Acknowledgment
The data of all idiopathic nephrotic syndrome patients were searched with the help of Qingsong Wu in the Medical Records Room.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Conolly ME, Wrong OM, Jones NF. Reversible renal failure in idiopathic nephrotic syndrome with minimal glomerular changes. Lancet. 1968;1(7544):665–668.
- Imbasciati E, Ponticelli C, Case N. Acute renal failure in idiopathic nephrotic syndrome. Nephron. 1981;28(4):186–191.
- International Study of Kidney Disease in Children. Nephrotic syndrome in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report. Kidney Int. 1978;13(2):159–165.
- Nolasco F, Cameron JS, Heywood EF, Adult-onset minimal change nephrotic syndrome: A long-term follow-up. Kidney Int. 1986;29(6):1215–1223.
- Jennette JC, Falk RJ. Adult minimal change glomerulopathy with acute renal failure. Am J Kidney Dis. 1990;16(5):432–437.
- Furuya R, Kumagai H, Ikegaya N. Reversible acute renal failure in idiopathic nephrotic syndrome. Intern Med. 1993;32(1):31–35.
- Grcevska L, Polenaković M. Minor degree of reversible renal insufficiency: A frequent complication of adult minimal-change nephrotic syndrome. Nephrol Dial Transplant. 1992;7(5):406–411.
- Bellomo R, Ronco C, Kellum JA, The ADQI workgroup: Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- Waldman M, Crew RJ, Valeri A, Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445.
- Smith JD, Hayslett JP. Reversible renal failure in the nephrotic syndrome. Am J Kidney Dis. 1992; 3:201–213.
- Lowenstein J, Schacht RG, Baldwin DS. Renal failure in minimal change nephrotic syndrome. Am J Med. 1981;70:227–233.
- Bagshaw SM, George C, Dinu I, A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210.
- Bell M, Liljestam E, Granath F, Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354–360.
- Hoste EA, Clermont G, Kersten A, RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73.
- Lin CY, Chen YC, Tsai FC, RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant. 2006;21:2867–2873.
- Ali T, Khan I, Simpson W, Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298.
- Chen T, Ding X, Chen B. Value of the RIFLE classification for acute kidney injury in diffuse proliferative lupus nephritis. Nephrol Dial Transplant. 2009;24(10):3115–3120.
- Dorhout Mees EJ, Roos JC, Boer P, Yoe OH, Simatupang TA. Observations on edema formation in the nephrotic syndrome in adults with minimal lesions. Am J Med. 1979;67:378.
- Robson AM, Giangiacomo J, Kienstra RA, Naqvi ST, Ingelfinger JR. Normal glomerular permeability and its modification by minimal change nephrotic syndrome. J Clin Invest. 1974;54:1190–1199.
- Hein A, Koomans. Pathophysiology of acute renal failure in idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2001;16:221–224.
- Vande Walle J, Mauel R, Raes A, ARF in children with minimal change nephrotic syndrome may be related to functional changes of the glomerular basal membrane. Am J Kidney Dis. 2004;43(3):399–404.
- Cameron MA, Peri U, Rogers TE, Minimal change disease with acute renal failure: A case against the nephrosarca hypothesis. Nephrol Dial Transplant. 2004;19:2642–2646.
- Andreucci M, Federico S, Andreucci VE. Edema and acute renal failure. Semin Nephrol. 2001;21(3):251–256.
- Cameron JS. The nephrotic syndrome and its complications. Am J Kidney Dis. 1987;10(3):157–171.
- Chamberlain MJ, Pringle A, Wrong OM. Oliguric renal failure in the nephrotic syndrome. Q J Med. 1966; 35(138):215–235.
- Vande Walle J, Donckerwolcke R, Boer P, Blood volume, colloid osmotic pressure and F-cell ratio in children with the nephrotic syndrome. Kidney Int. 1996;49:1471–1477; doi:10.1038/ki.1996.207.
- Dorhout Mees EJ, Geers AB, Koomans HA. Blood volume and sodium retention in the nephrotic syndrome: A controversial pathophysiological concept. Nephron. 1984;36:201–211.
- Geers AB, Koomans HA, Boer P, Plasma and blood volumes in patients with the nephrotic syndrome. Nephron. 1984;38:170–173.
- Vande Walle JG, Donckerwolcke RA, van Isselt JW, Volume regulation in children with early relapse of minimal-change nephrosis with or without hypovolaemic symptoms. Lancet. 1995;346(8968):148–152.