Abstract
Patients with gout often have concurrent chronic kidney disease (CKD); the relationship between the two conditions is still unclear. Previous studies have identified an association between low level of urinary uromodulin (UMOD) and CKD within the setting of diabetes and lupus. The aim of this study was to examine the association between urinary UMOD excretion and CKD in patients with gout. A total of 53 Taiwanese gout patients with stable disease activity were enrolled. Patients were divided into a CKD group (n = 25) and a non-CKD group (n = 28). Using Pearson correlation analysis, urinary UMOD excretion was positively correlated with estimated glomerular filtration rate (Ha: ρ > 0, p = 0.004). Using multivariate analysis, patients with CKD and gout were associated with lower urinary UMOD excretion than those who have gout alone [odds ratio (95% CI): 0.826 (0.694–0.985), p < 0.001]. Patients with CKD and gout were also more likely to be older (p < 0.001) and have higher uric acid levels (p < 0.001). This study implicates that UMOD might play a role in the relationship between gout and CKD. Further studies with animal models of gout and CKD would be recommended.
INTRODUCTION
Gout is a condition characterized by the deposition of monosodium urate crystals in joints and adjacent soft tissues with associated inflammation and arthritis. Chronic kidney disease (CKD) often accompanies gout. However, the relationship between gout and CKD is not completely understood; the interplay between the two pathologies remains to be a chicken-and-egg debate. Previous studies reported that urinary uromodulin (UMOD) excretion was lower in patients with diabetic nephropathy,Citation1 polycystic kidney disease,Citation2,Citation3 lupus nephritis,Citation4 familial juvenile hyperuricemic nephropathy,Citation5 and medullary cystic kidney disease.Citation5 The authors believe that UMOD may play a role in the dynamics between CKD and gout.
The Tamm–Horsfall protein (THP), also known as UMOD, is an 80-kDa renal tubular glycoprotein that is abundant in human urine. UMOD is synthesized exclusively in the cells of thick ascending limb (TAL) of the Henle's loop, with the exception of the macula densa. The function of UMOD is not completely understood. However, it is implicated in the pathogenesis of various disorders of the distal nephrons and the urinary tract, such as various nephropathies, urolithiasis, and tubulointerstitial nephritis.Citation6 In addition, UMOD is an important urinary protective factor against stone formation and urinary tract infection, particularly those caused by Escherichia coli.Citation7,Citation8 The association between UMOD and CKD has attracted much interest; previous studies have shown that urinary UMOD is decreased in patients with CKD.Citation1–4,Citation9,Citation10 However, not all patients with CKD have decreased urinary UMOD excretion. Thornley et al.Citation11 demonstrated that patients with CKD had a greater range of urinary UMOD excretion than normal subjects. Further, Prajczer et al.Citation12 recently presented new data showing 22% of study patients with CKD had normal urinary UMOD levels. Therefore, we wanted to investigate whether a relationship between urinary UMOD and CKD also exists in the setting of gout. To the best of our knowledge, there has been no such previous study.
MATERIALS AND METHODS
Patients
Patients who were previously diagnosed with gout and were followed up regularly at the rheumatology and nephrology clinic at Kaohsiung Chang Gung Memorial Hospital were prospectively enrolled between 1 January 2008 and 31 December 2009. Exclusion criteria included pregnancy, less than 16 years of age, CKD stage 5, recent acute gout attacks (within 3 months from the time of most recent visit to the clinics), regular use of non-steroidal anti-inflammatory drugs, presence of a known heritable illness transmitted as an autosomal dominant trait, and family history of CKD. A total of 53 patients were enrolled.
Definition of CKD
The glomerular filtration rate (GFR) was estimated from Modification of Diet in Renal Disease abbreviated formula:Citation11 186 × (serum creatinine)−1.154 × (age)−0.203 × (0.742 if female). An eGFR less than 60 mL/min/1.73 m2 for at least 3 months was used to define CKD.
Methods
Enrolled patients were divided into a non-CKD group and a CKD group. All participants provided informed consent and the study protocol was approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital. Urine and blood samples were obtained from individuals at the outpatient clinic and were examined for urinary creatinine (UCR), UMOD, serum uric acid (UA), and serum creatinine. Demographic data, such as diabetes mellitus (DM), hypertension, and smoking history, were recorded from the medical charts.
Urinary UMOD Measurement
An enzyme-linked immunosorbent assay (ELISA) was used to measure urinary UMOD as previously described by Kobayashi and FukuokaCitation13 but with several modifications. The 96-well ELISA plates were coated with 100 µL/well of anti-THP antibody (Sheep-Anti-Human Tamm–Horsfall Glycoprotein, AbD, Oxford, UK) in phosphate-buffered saline (PBS) at 10 µg/mL. PBS with 0.1% Tween 20 – polyoxyethylenesorbitanmonolaurate (PBS-Tween) was used as a washing buffer. Plates were incubated at 4°C overnight. To prevent non-specific adherence to the plates, 25% fetal calf serum (FCS) in PBS (pH 7.2) was added as a blocking agent. After incubation for 1 h at room temperature, wells were aspirated and washed five times with more than 300 µL/well of PBS-Tween buffer and then the plate was inverted and blotted on absorbent paper to remove residual buffer. Urine samples were first diluted 1:250 in a dilution buffer, then twofold dilutions were prepared and applied as 100 μL aliquots to the ELISA. Baseline controls (dilution buffer alone) and THP standards (Human Tamm–Horsfall Glycoprotein, ChemiCON, International, Inc., Temecula, CA, USA) were included on each plate.
After a 2 h incubation period at 37°C, plates were washed five times with more than 300 µL/well of PBS-Tween. Then 100 μL of a HRP-labeled anti-THP antibody solution, diluted 1:3000 with 25% FCS in PBS, was added to each well followed by incubation for 2 h at 37°C. After five washes with PBS-Tween, 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate solution was added to the plates. The color reaction was stopped by the addition of 50 μL of 1N H2SO4 at 30 min. Immediately afterward, absorbance at 450 nm was measured to determine product concentration and at 620 nm for estimation of background absorbance and scattering. A THP standard curve was determined for each plate. THP concentration was calculated from multiple dilutions of each sample.
Calculation of Urinary UMOD/UCR Ratio to Determine Daily UMOD Excretion
Daily urinary UMOD excretion was expressed as the urinary UMOD/UCR ratio to correct for different levels of patient hydration.
UMOD Genotyping
Ten patients (25% of the study population) with very low urinary UMOD [urinary UMOD/UCR (mg/g) < 1] received UMOD genotyping. Blood samples from patients with very low urinary UMOD levels were sent for genetic analysis. Samples were spun and the buffy coats were extracted to perform polymerase chain reaction and DNA sequencing of the UMOD coding sequence (exons 2–12) as previously reported by Hart et al.Citation5
Statistical Analysis
Statistical analysis was performed with PASW version 18 for Windows (SPSS, Inc., Chicago, IL, USA). The relationship of eGFR and the urinary UMOD/UCR ratio was assessed by Pearson's correlation coefficient. Baseline variables, including continuous data and categorical data, were compared for the CKD group and the non-CKD group. All results are expressed as means ± standard deviations for continuous data and as percentages for categorical data. Fisher's exact test was used to test the compared categorical data of the CKD group and the non-CKD control group. An independent t-test was used to compare continuous variables between the two groups. Stepwise logistic regression was used to assess the impact of variables on the occurrence of CKD in patients with gout. Significant variables (age, UA, urinary UMOD/UCR ratio, hypertension, DM, and smoking status) were included in the logistic regression model. A p-value of less than 0.05 was considered statistically significant. All tests were two-tailed.
RESULTS
Genotyping of UMOD Gene
UMOD genotyping was performed on 10 patients (25% of the study population) who had very low urinary UMOD [urinary UMOD/UCR (mg/g) < 1]. No UMOD gene mutation was identified.
Correlation of Urinary UMOD/UCR and eGFR in Patients with Gout
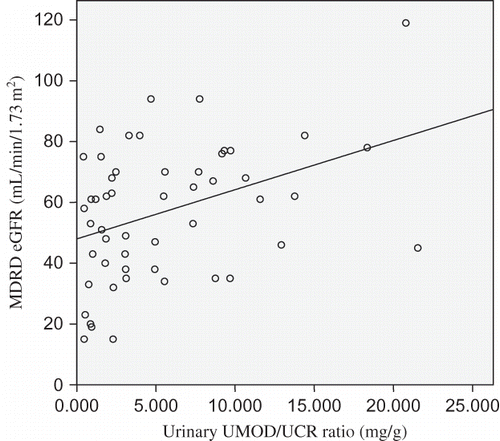
First, we used the Pearson correlation analysis to assess the relationship between the urinary UMOD/UCR ratio and eGFR in all 53 gout patients. Our results indicate that the urinary UMOD/UCR ratio was positively correlated with eGFR () (Ha: ρ > 0, p = 0.004).
Association of Categorical and Continuous Variables with CKD
shows the clinical characteristics of gout patients with and without CKD. There were significant differences between the two groups in age and serum UA (p < 0.05 for both). Other variables, such as gender, hypertension, DM, smoking, and urinary UMOD/UCR ratio, were not significantly different between the two groups.
Table 1. Comparison of demographic data, age, urinary UMOD excretion, serum uric acid, serum creatinine, and eGFR in gout patients with and without CKD
Multivariate Analysis by Stepwise Logistic Regression
Finally, we used logistic regression to determine whether serum UA, age, and urinary UMOD/UCR ratio were associated with CKD. Risk factors, including hypertension, DM, age, serum UA, and urinary UMOD /UCR ratio, were further examined by multivariate analysis (). Our results indicate that age, urinary UMOD/UCR ratio, and serum UA were significantly associated with CKD (p < 0.001 for all). Elevated UA was the best predictor of CKD and urinary UMOD excretion was negatively correlated with CKD (p < 0.001). Hypertension, DM, and smoking had no significant association with CKD.
Table 2. Multivariate analysis using a stepwise logistic regression model to predict variables associated with CKD in gout patients
DISCUSSION
Based on our results and the results of previous studies, multiple comorbid conditions are associated with CKD in gout patients, including hypertension, metabolic syndrome, and DM.Citation14 These complicate the management of gout.Citation15 Moreover, the potential role of hyperuricemia in the pathogenesis of kidney disease has become the subject of intense interest and speculation. However, the pathogenesis of CKD in the presence of gout remains incompletely understood. Clinically, many patients have primary renal disease at the time of or prior to the first attack of gout. Recent studies have reported that mild hyperuricemia can be induced in normal rats by the uricase inhibitor (oxonic acid), leading to hypertension, intra-renal vascular disease, and renal injury.Citation16 Urate crystal nephropathies are common in patients with gout, with frequencies of 79–99% based on autopsy studies.Citation17,Citation18 Therefore, other mechanisms need to be considered to fully elucidate the relationship between gout and CKD.
Previous studies reported that urinary UMOD excretion was lower in patients with diabetic nephropathy,Citation1 polycystic kidney disease,Citation2,Citation3 lupus nephritis,Citation4 familial juvenile hyperuricemic nephropathy,Citation5 and medullary cystic kidney disease.Citation5 The present study is the first to report a relationship between urinary UMOD excretion and CKD in gout patients. In our study of 53 Taiwanese patients with gout, we found that the urinary UMOD/UCR ratio was positively correlated with eGFR. Urinary UMOD level was also lower in the group with both CKD and gout. The authors believe that these add further insight into the relationship between CKD and gout. Therefore, a tentative mechanism is offered below.
First, it is known that UMOD leakage can cause inflammation of renal tissue and a previous study reported that damage to the TAL and cells distal to the TAL results in UMOD leakage into the interstitium and subsequent inflammation. Sustained leakage of UMOD into the interstitium would likely cause an immune reaction against UMOD-secreting cells, resulting in decreased number of TAL cells and eventually decreased urinary UMOD excretion.Citation12 Second, chronic urate nephropathy leads to precipitation of UA in the renal medulla, and this is accompanied by formation of tophi at the cortico-medullary junction and deep in the medulla. The characteristic tophi associated with gout are believed to provoke an inflammatory response that leads to fibrosis, nephropathy, and ultimately to chronic and irreversible renal failure.Citation19 Third, UMOD is produced exclusively in the TAL cells of the nephron and is targeted by glycosylphosphatidylinositol in the apical membrane.Citation20 Here, the protein is cleaved (most likely by proteolysis) and is released into the lumen.Citation21 Therefore, we can speculate that in patients with gout, tophi may precipitate around TAL and set up an initial inflammation which damages the cells of TAL. This subsequently leads to leakage of UMOD and further inflammation that persists in the vicious cycle. Chronic inflammation will eventually lead to depletion of TAL cells and sclerosis of tissue, causing a drop in urinary UMOD.
Another possibility is that UMOD acts as a protective factor against the renal damage from tophi deposition. Patients with lower urinary UMOD excretion would therefore be at a higher risk for chronic renal impairment.
In conclusion, the present study reports that urinary UMOD excretion was negatively correlated with CKD in gout patients. The authors believe that this relationship shed light on the dynamics between CKD and gout. Further, UMOD may have the potential to be used both as a predictive marker and as a therapeutic agent for CKD in patients with gout. We suggest that future studies with animal models should be carried out to further elucidate and confirm the role of UMOD in the complex relationship between CKD and gout.
ACKNOWLEDGMENTS
We thank our patients for contributing samples for this study. The technical assistance from Miss Ming-Chi Yang is deeply appreciated. This study was supported by Chang Gung Memorial Hospital CMRP grant number CMRPG850521.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sejdiu I, Torffvit O. Decreased urinary concentration of Tamm-Horsfall protein is associated with development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetic patients. Scand J Urol Nephrol. 2008;42:168–174.
- Torffvit O, Jorgensen PE, Kamper AL, Urinary excretion of Tamm-Horsfall protein and epidermal growth factor in chronic nephropathy. Nephron. 1998;79:167–172.
- Lynn KL, Marshall RD. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clin Nephrol. 1984;22:253–257.
- Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85:207–214.
- Hart TC, Gorry MC, Hart PS, Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricemic nephropathy. J Med Genet. 2002;39:882–892.
- Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: Biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676.
- Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004;66:1159–1166.
- Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–F802.
- Chakraborty J, Below AA, Solaiman D. Tamm-Horsfall protein in patients with kidney damage and diabetes. Urol Res. 2004;32:79–83.
- Bleyer AJ, Hart TC, Shihabi Z, Robins V, Hoyer JR. Mutations in the uromodulin gene decrease urinary excretion of Tamm-Horsfall protein. Kidney Int. 2004;66:974–997.
- Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: Urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond). 1985;68:529–535.
- Prajczer S, Heidenreich U, Pfaller W, Kotanko P, Lhotta K, Jennings P. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol Dial Transplant. 2010;25:1896–1903.
- Kobayashi K, Fukuoka S. Conditions for solubilization of Tamm-Horsfall protein/uromodulin in human urine and establishment of a sensitive and accurate enzyme-linked immunosorbent assay (ELISA) method. Arch Biochem Biophys. 2001;388:113–120.
- Richette P, Gout BT. Lancet. 2010;375:318–328.
- Gaffo AL, Saag KG. Management of hyperuricemia and gout in CKD. Am J Kidney Dis. 2008;52:994–1009.
- Kang DH, Nakagawa T, Feng L, A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897.
- Tarng DC, Lin HY, Shyong ML, Wang JS, Yang WC, Huang TP. Renal function in gout patients. Am J Nephrol. 1995;15:31–37.
- Kang DH, Nakagawa T. Uric acid and chronic renal disease: Possible implication of hyperuricemia on progression of renal disease. Semin Nephrol. 2005;25:43–49.
- Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB. Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis. 1999;33:225–234.
- Rindler MJ, Naik SS, Li N, Hoops TC, Peraldi MN. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem. 1990;265:20784–20789.
- Cavallone D, Malagolini N, Serafini-Cessi F. Mechanism of release of urinary Tamm-Horsfall glycoprotein from the kidney GPI-anchored counterpart. Biochem Biophys Res Commun. 2001;280:110–114.