Abstract
Objective: To investigate the effects of norcantharidin (NCTD) on tubulointerstitial fibrosis of diabetic nephropathy (DN) in streptozotocin-induced rat model. Methods: Sprague–Dawley rats were randomly divided into control group, model group, low-dose NCTD (0.05 mg/kg/day) group, and high-dose NCTD (0.1 mg/kg/day) group. The model group was induced by injection intraperitoneally with 30 mg/kg streptozotocin in 0.1 mol/L sodium citrate solution (pH 4.5), after high-calorie foods were given for 2 months. NCTD was administered daily after the DN rat model was built. Rats were sacrificed at the end of the third and the eighth week; renal fibrosis and the expression of FN, collagen IV, TGF-β1, and calcineurin (CaN) were detected by Masson and immunohistochemistry staining, respectively. Results: Tubulointerstitial fibrosis was observed in DN rats, this kind of pathological changes was ameliorated in NCTD treatment group (p < 0.05). The expressions of FN, collagen IV, and TGF-β1 protein increased in the tubulointerstitial field of DN rats compared with the rats in control group. NCTD treatment could dose-dependently decrease their expression and reverse the fibrotic degree (p < 0.05). Meanwhile, the expression of CaN was detected in tubular fields of normal kidney and increased in the tubulointerstitial field in DN rats. However, NCTD downregulated its expression in a dose-dependent manner (p < 0.05). Conclusions: NCTD could downregulate FN, collagen IV, and TGF-β1 expression in tubulointerstitial fields and attenuate tubulointerstitial fibrosis in the early stage of DN rats. NCTD also alleviated the expression of CaN in tubules in DN. The relationship between the role of NCTD's anti-tubulointerstitial fibrosis and its inhibition to CaN expression remains to be further elucidated.
INTRODUCTION
Glomerulosclerosis has long been considered to be the primary lesion and main manifestation in diabetic nephropathy (DN) followed by a similar fibrosing process in the tubulointerstitial region. However, a recent study reported that tubulointerstitial fibrosis could also occur in the early stage of DN and directly induce deterioration of renal function, independent of the glomerular lesions.Citation1,Citation2 Unfortunately, active control of blood glucose and lipid level, decreasing proteinuria by blocking the renin–angiotensin–aldosterone system, and ameliorating insulin resistance cannot effectively inhibit tubulointerstitial fibrosis of DN.Citation3 And, so far, there has not been an ideal drug which can reverse tubulointerstitial fibrosis and alleviate the decline of renal function in DN.
Norcantharidin (NCTD) is a synthetic derivative of cantharidin. Our previous studies first reported that NCTD could reduce renal interstitial fibrosis in the rat models of protein overload nephropathyCitation4 and obstructive nephropathy (data not shown). In in vitro studies, we found that NCTD could block the TGF-β1-mediated renal tubular epithelial cells' transdifferentiation.Citation5 We hypothesize that NCTD is a promising drug to inhibit tubulointerstitial fibrosis. This study was designed to observe the effect of NCTD on tubulointerstitial fibrosis in the rat model with DN, and it was hoped to provide a theoretical basis for the development of prospective drugs to delay the progression of renal function in DN.
MATERIALS AND METHODS
Experimental DN Model
Forty-four healthy 8-week-old male Sprague–Dawley rats, weighting 165 ± 12.5 g (SPF, Certification number ×2003115403), were purchased from Central South University Xiangya School of Medicine Animal Department. The rats were fed with high-sugar and high-fat diet for 8 weeks before intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO, USA) at a dose of 30 mg/kg. High-sugar and high-fat diet was prepared by the Experimental Animal Center, Second Xiangya Hospital, including 18% of the lard, 20% sugar, 3% egg yolk, and 59% basic stoyer. After 72 h of streptozotocin injection, blood glucose and urine glucose tests were given. The establishment of diabetes model was considered successful if fasting blood glucose was greater than 13.8 mmol/L, random blood glucose was higher than 16.7 mol/L, and urine glucose was ++++. And the DN model was considered to be established successfully if urine protein was more than 30 mg/24 h after the diabetes model's establishment.Citation6 During the experiment, tail vein blood glucose was measured every 3 days. Certain amount of insulin (0.4–3.2 U) was injected in rats whose blood glucose was higher than 26.0 mmol/L; random blood glucose was maintained above 16.7 mmol/L. Body weight was measured once a week. Rats with DN were randomly divided into three groups that were either untreated or treated with low-dose NCTD (0.05 mg/kg/day) or high-dose NCTD (0.1 mg/kg/day)Citation4 (n = 12 in each group). An additional set of rats fed with normal diet and given the same dose of intraperitoneal injection of citrate buffer was served as the control group (n = 8). All the rats were sacrificed in the third week and eighth week separately. The animal care protocols in this study were approved in advance by an appropriate animal care and use committee.
Urine and Blood Measurements
The rats were placed in metabolic cages with free access to water. Twenty-four-hour urine samples were collected for urinary protein quantitation using the Bio-Rad method (Shanghai Sun Biological Company, Shanghai, China). Blood samples were obtained from the abdominal aorta at sacrifice and used for measuring blood creatinine.
Masson Staining in Kidney
The 2–3 µm thick paraffin sections were conventionally dewaxed from different levels of ethanol to water. Hematoxylin was used to stain nucleus for 3 min at room temperature, and the sections were rinsed with fast running water for 30–60 s. After that, Ponceau acid fuchsin solution was used to dye sections for 30–60 min and tap water was used to rinse for 1 min. Then the sections were dyed consequently in 1% phosphorus–molybdate for 1 min and in the 2% solution of aniline blue for 3–5 min without washing. After that, all the sections were washed with water and dehydralized with gradient alcohol. Then xylene was used for 1 min to make all the sections transparent and then all the sections were enclosed with neutral gum. Collagen fibers were stained blue. Microscope (OLYMPUS CX41, Tokyo, Japan) was used to observe and photograph all the sections. In each section, 10 non-overlapping interstitial visions were collected. HMIAS-2000 high-definition color medical analysis system was used for automatic measurement and analysis, and the percentage of tubulointerstitial area in the total vision in each section was calculated.
Immunohistochemistry Staining for the Expressionof FN, Collagen IV, TGF-β1, and Calcineurin Proteinin Kidney
Paraffin-embedded tissue sections were dewaxed, hydrated, treated with 5 mmol/L levamisole for blocking endogenous alkaline phosphatase, and incubated with blocking serum for 30 min at room temperature to reduce nonspecific background staining. Sections were rehydrated in PBS including 0.1% BSA for 15 min before addition of the appropriate blocking serum for an additional 15 min. Sections were incubated with rabbit anti-rat FN, TGF-β1 polyclonal antibody 1:50 (Santa Cruz, Inc., California, UK), rabbit anti-rat collagen IV polyclonal antibody 1:50 (Abcam, Inc., London, UK), and rabbit anti-rat calcineurin (CaN) polyclonal antibody 1:100 (Cell Signaling, Inc., Danvers, MA, USA) overnight at 4°C. After rinsing, the sections were incubated with biotinylated goat anti-rabbit IgG (Abcam, Inc.) and processed using an alkaline phosphatase-streptavidin-biotin immunoperoxidase method (Maixin Biotechnological Company, Shenzhen, China). The tissue sections were counterstained with hematoxylin. Negative controls for specific labeling were performed in parallel by replacing the primary antibody with a normal rabbit serum. All the images were semi-quantitatively analyzed. The images were observed under the 100× light microscope. In each group, 10 vision without glomerular and tubulointerstitial artery views were observed. The Image-Pro P1uS Version 6.0 image analysis system was used to measure the percentage of the optical density (OD) of the view of positive OD and positive area. The average OD(AOD)/area was used on behalf of the expression degree of respective index.
Statistical Analysis
SPSS13.0 software was used for statistical analysis. Data are expressed as the mean ± SD values; p < 0.05 was considered to be statistically significant. All variables were compared using an ANOVA or Student's t-test.
RESULTS
The Effect of NCTD on the General Condition, Urinary Protein, and Serum Creatinine of Rat Models with DN
There was no difference of the general condition and the other clinical indexes between the control group and DN group except for the increased blood glucose at the third week. After 8 weeks, the DN rats suffered from worse spirit, slower growth, less shiny hair, and significantly increased toe infection compared with normal rats. Also, the DN rats showed significant polydipsia, polyphagia, polyuria, and other symptoms. Compared with the normal control group, the body weight of DN rats was significantly reduced. Meanwhile, the kidney was significantly bigger and heavier, and the kidney weight/body mass index was obviously larger, compared with the control group (p < 0.05). Twenty-four-hour urinary albumin excretion rate (UAER) and serum creatinine concentration (Scr) in DN model group were significantly higher than those in normal control group (p < 0.05). And there was no significant difference in UAER, Scr, and blood glucose level between NCTD treatment group and DN group (p > 0.05), but the general condition was markedly improved. There were no signs of infection, and increase in kidney volume was not as large as that in DN group (p < 0.05). But still, the body weight was significantly reduced compared with the normal group (p > 0.05). Kidney weight/body mass index in NCTD (0.1 mg/kg/day) group was reduced compared with diabetic group (p < 0.05). At the end of the eighth week, four rats in model group, three in NCTD (0.05 mg/kg/day) group, and two in NCTD (0.1 mg/kg/day) group died. The cause of death was estimated to be infection induced by high blood glucose and metabolism disorder leading to organ failure (see ).
Table 1. The general condition and urine and blood indexes of rats in different groups
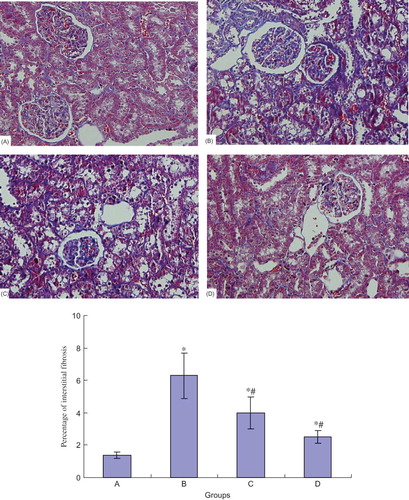
Masson Staining of the Kidney in Different Groups
There was no tubular expansion, interstitial infiltration of inflammatory cells, and fibrous proliferation in normal control group. At the end of the eighth week, part of the kidney samples in the model group showed enlargement of glomerular volume and increase of mesangial matrix. Proximal tubular epithelial cells' brush border decreased or disappeared, and in some renal tubular epithelial cells vacuolar degeneration or even falling off was observed. A small amount of interstitial fibrosis could be observed. Blue-stained areas by Masson staining mainly represent collagen, and the degree of interstitial fibrosis can be inferred by determining blue-stained interstitial areas. According to the examination results, renal tubular injury and interstitial fibrosis in NCTD group at the end of the eighth week were slightly ameliorated, especially in high-dose NCTD (0.1 mg/kg/day) group, compared with the DN group (see ).
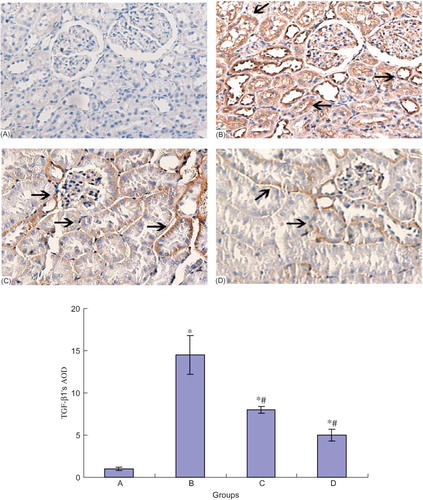
The Expression of FN, Collagen IV, and TGF-β1 Protein in Different Groups
FN and collagen IV mainly deposited on the glomerular and tubular basement membrane of the rats in normal control group. Their expression significantly increased in the tubules of DN rats (p < 0.05). NCTD dose-dependently downregulated the expression of tubular FN and collagen IV protein (p < 0.05) (see and ).
Figure 2. FN protein expression of the kidney in different groups (×100). *p < 0.05 compared with normal group, #p < 0.05 compared with DN group. (A) Control group; (B) DN group; (C) DN + NCTD (0.05 mg/kg/day) group; (D) DN + NCTD (0.1 mg/kg/day) group.
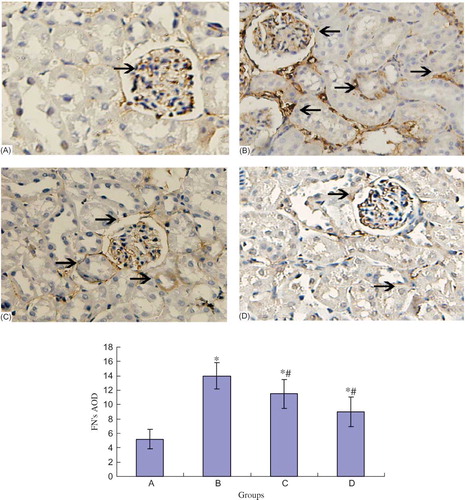
Figure 3. Collagen IV protein expression of the kidney in different groups (×100). *p < 0.05 compared with normal group, #p < 0.05 compared with DN group. (A) Control group; (B) DN group; (C) DN + NCTD (0.05 mg/kg/day) group; (D) DN + NCTD (0.1 mg/kg/day) group.
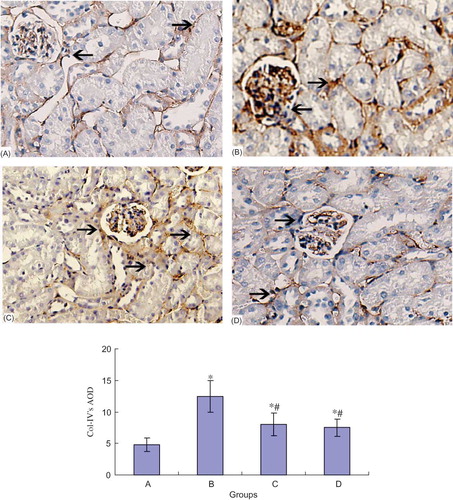
TGF-β1 had a small amount of expression in the normal collecting duct, distal tubule, and proximal tubule cells. In DN group, TGF-β1 expression in the above regions increased (p < 0.05). However, NCTD could reduce the TGF-β1 expression in a dose-dependent manner (p < 0.05) (see ).
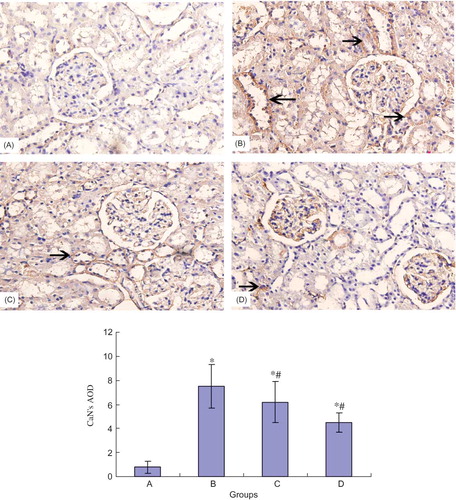
The Expression of CaN Protein in Different Groups
Immunohistochemical staining showed that there was only weak expression of CaN in normal rat's glomerule and tubule. At the end of the eighth week, CaN expression of renal tubule in model group was significantly increased compared with that in normal group. NCTD obviously decreased the CaN expression in renal tubule (see ).
DISCUSSION
DN is a major chronic complication of diabetes with a growing incidence, which has become the main reason leading to end-stage renal failure. In diabetes, glomerular fibrosis is observed in the early progression from incipient to overt nephropathy, whereas presently it has been reported that tubulointerstitial fibrosis could also present itself in these early stages.Citation6–8 Parving et al.Citation9 described a high prevalence of nondiabetic glomerular diseases in Danish T2DM patients with proteinuria undergoing clinical renal biopsies; only 23% had a variety of glomerulopathies. Similarly, Gambara et al.Citation10 found that 33% of proteinuric T2DM patients had glomerular disease superimposed on diabetic glomerulosclerosis. Therefore, a substantial subset of type 2 diabetic patients, despite the presence of micro-albuminuria or proteinuria, have normal glomerular structure with tubulointerstitial abnormalities. It had been demonstrated that tubulointerstitial disease might be more important in the progression from moderate renal insufficiency to end-stage renal disease, independent of glomerular lesion. Elucidating the mechanism of interstitial fibrosis, exploring the effective therapeutic measures will be of great significance for delaying the progression of renal failure. Unfortunately, there are no ideal drugs which can fully reverse the diabetic renal interstitial fibrosis.
Traditional Chinese medicine has the advantages of fewer side effects, safety and multitarget to play a pharmacodynamics role, from which, discovering drugs against renal fibrosis will be of important meaning. NCTD is a synthetic cantharidin derivative; with the removal of the two methyl groups at the 2 and 3 positions of cantharidin, its renal toxicity is significantly reduced.Citation11 It has become a kind of widely used antitumor drug with China's independent intellectual property rights.Citation12–14 Our previous study found that it has an anti-tubulointerstitial fibrosis function in protein overload nephropathy modelsCitation4 and obstructive nephropathy models (data not shown). This study was designed to observe the effect of NCTD on renal fibrosis in DN nephropathy.
High-sugar and high-fat diet with low-dose streptozotocin injection induced insulin resistance and impaired β-cell function, leading to elevated blood sugar, lipids, and renal function abnormalities, which was similar to human type 2 diabetes and kidney damage phenotype. In our study, blood glucose in DN rats was significantly higher than that in normal control group at the eighth week, and the rats presented obvious diabetic symptoms including polydipsia, polyphagia, polyuria, and weight loss. Urinary albumin was significantly increased. Renal pathology showed proximal tubule epithelial cell loss. All these results showed that the construction of DN model was successful.
Histologically, tubulointerstitial fibrosis in DN is characterized by an accumulation of extracellular matrix (ECM) proteins such as FN, collagen in tubular interstitium. TGF-β1 as the predominant agent can mediate the ECM accumulation in the fibrotic interstitium and it is likely a candidate in the development and progression of fibrotic complications observed in DN. Our study showed that obvious renal interstitial fibrosis could be observed at the eighth week by Masson staining. Aside from it, immunohistochemistry results showed that the expression of collagen IV and FN around tubule increased, and TGF-β1 expression was also markedly enhanced. All of these demonstrated that animal models presented the appearance of interstitial fibrosis.
However, NCTD could reduce the relative area of renal interstitial collagen, downregulate the expression of collagen IV and FN around tubule, and also decrease the expression of TGF-β1. These results suggest that NCTD could alleviate fibrosis of the type 2 DN rats at the early stage. NCTD, as an anti-fibrosis drug, has a promising prospect. Because of the profibrotic actions of TGF-β, it was made an ideal therapeutic target for renoprotective agents. Inhibition of TGF-β might suppress the expression of genes encoding for the ECM in interstitium. There was a report that chronic treatment with TGF-β-neutralizing antibodies markedly diminished the expression of collagen and FN and reduced mesangial matrix expansion in DN.Citation2 We do not know whether the decrease of FN and collagen IV expression is the result of the inhibition of NCTD on TGF-β1 expression.
In this study, we found another interesting phenomenon: it was demonstrated that CaN expression in tubules significantly increased in DN model rats. And NCTD in a dose-dependent manner reduced the expression of CaN in the region of renal tubulointerstitium. It has been reported that NCTD is an inhibitor of protein phosphatase.Citation15 Baba et al.Citation16,Citation17 found that NCTD can inhibit the activity of CaN. Another research found that there is only a small amount of CaN expression in a normal kidney, mainly in renal proximal tubules.Citation18 Gooch et al.Citation19,Citation20 reported that CaN expression in kidney tissue increased, and the activity of CaN phosphatase also upregulated in the early stage of diabetes. CaN played an important role in glomerulosclerosis of DN, and inhibiting CaN could obviously alleviate the degree of glomerulosclerosis.Citation21,Citation22 However, whether CaN contributes to the renal interstitial fibrosis in DN is unknown. Presently, we are investigating the relationship of NCTD's downregulating the expression of CaN and NCTD's anti-renal interstitial fibrosis in DN.
In summary, it can be suggested that NCTD might be a prospective and useful drug to prevent the tubulointerstitial fibrosis in diabetes. In future studies, it is needed to focus on the possible toxicity and side effects of this compound and the molecular mechanisms of action.
Acknowledgments
This study was supported by a specialized Research Fund for the Doctoral Program of Higher Education of China (Grant no. 20070533062), the Natural Science Foundation of Hunan Province, China (Grant no. 10JJ2011), and the scientific project of Research Center of Metabolic Syndrome in Central South University of China (Grant no. DY-2008-02-03).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Magri CJ, Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med. 2009;20(6):551–555.
- Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol. 2010;31(1):68–74.
- Balakumar P, Arora MK, Ganti SS, Recent advances in pharmacotherapy for diabetic nephropathy: Current perspectives and future directions. Pharmacol Res. 2009; 60(1):24–32.
- Liu FY, Li Y, Peng YM, Noncantharidin ameliorates proteinuria, associated tubulointerstitial inflammation and fibrosis in protein overload nephropathy. Am J Nephrol. 2008;28(3):465–477.
- Liu FY, Sun Y, Sun L, Noncantharidin inhibits tubular epithelial mesenchymal transition by downregulating activation of Smad2/3 and snail. ASN Renal Week. 2009;abstract (number: 550493).
- Katz A, Caramori ML, Sisson-Ross S, An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002; 61(6):2058–2066.
- Tervaert TW, Mooyaart AL, Amann K, Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563.
- Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27(2):195–207.
- Parving H-H, Gall M-A, Skøtt P, Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758–762.
- Gambara V, Mecca G, Remuzzi G, Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3(8):1458–1466.
- Massicot F, Dutertre-Catella H, Pham-Huy C, In vitro assessment of renal toxicity and inflammatory events of two protein phosphatase inhibitors cantharidin and nor-cantharidin. Basic Clin Pharmacol Toxicol. 2005;96(1):26–32.
- Huang Y, Liu Q, Liu K, Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59(3):209.
- Huang Y, Liu Q, Liu K, Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59(3):201–208.
- Hill TA, Stewart SG, Ackland SP, Norcantharimides, synthesis and anticancer activity: Synthesis of new norcantharidin analogues and their anticancer evaluation. Bioorg Med Chem. 2007;15(18):6126–6134.
- Bertini I, Calderone V, Fragai M, Structural basis of serine/threonine phosphatase inhibition by the archetypal small molecules cantharidin and norcantharidin. J Med Chem. 2009;52(15):4838–4843.
- Baba Y, Hirukawa N, Tanohira N, Structure-based design of a highly selective catalytic site-directed inhibitor of Ser/Thr protein phosphatase 2B (calcineurin). J Am Chem Soc. 2003;125(32):9740–9749.
- Baba Y, Hirukawa N, Sodeoka M. Optically active cantharidin analogues possessing selective inhibitory activity on Ser/Thr protein phosphatase 2B (calcineurin): Implications for the binding mode. Bioorg Med Chem. 2005;13(17):5164–5170.
- Tumlin JA, Someren JT, Swanson CE, Expression of calcineurin activity and -subunit isoforms in specific segments of the rat nephron. Am J Physiol Renal Physiol. 1995;269(4 pt. 2):F558–F563.
- Gooch JL. An emerging role for calcineurin A in the development and function of the kidney. Am J Physiol Renal Physiol. 2006;290(4):769–776.
- Cobbs SL, Gooch JL. NFATc is required for TGF beta-mediated transcriptional regulation of fibronectin. Biochem Biophys Res Commun. 2007;362(2):288–294.
- Gooch JL, Barnes JL, Garcia S, Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal. 2003;284(1):144–154.
- Gooch JL, Gorin Y, Zhang BX, Involvement of calcineurin in transforming growth factor-β mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004; 279(15):15561–15570.