Abstract
Background: Peritoneal fibrosis is a common complication of long-term peritoneal dialysis (PD) and is the main cause of dialysis inadequacy and PD withdrawal. It has been reported that Tanshinone IIA can ameliorate fibrosis in various tissues. In this report, we investigate the effects of Tanshinone IIA on peritoneal fibrosis in an animal model. Methods: Forty rats were randomly divided into four groups (n = 10 per group) that received daily intraperitoneal injection of saline, 4.25% glucose-based peritoneal dialysis fluid (PDF), or PDF along with 50 or 100 mg/L Tanshinone IIA. Eight weeks later, the rats were sacrificed and peritoneal tissue samples were collected for analysis. The expression of transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) in parietal peritoneum was examined by immunohistochemistry. The mRNA and protein expression of TGF-β1 and CTGF in omentum was examined by reverse transcription polymerase chain reaction or Western blot. Results: Tanshinone IIA significantly suppressed submesothelial compact zone thickening and matrix accumulation induced by 4.25% glucose-based PDF. Tanshinone IIA also reduced TGF-β1 and CTGF expression in parietal peritoneum as well as in omentum in a dose-dependent manner. Conclusion: Tanshinone IIA prevented the progression of peritoneal fibrosis in this rat model. Tanshinone IIA may be a novel therapy for peritoneal fibrosis in patients undergoing long-term PD.
INTRODUCTION
Peritoneal dialysis (PD) is an effective renal replacement method for patients with end-stage renal disease. However, peritoneal fibrosis after long-term PD therapy is thought to be the major cause of ultrafiltration failure and PD withdrawal.Citation1–3 Although the precise mechanism of the pathogenesis of peritoneal fibrosis is not clear, it is generally speculated that daily instillation of bio-incompatible peritoneal dialysis fluid (PDF) sustains the development of morphological and functional changes.Citation4–6
Several growth factors, such as transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF), have been shown to play key roles in the development of fibrosis. TGF-β1 has been demonstrated to be the most important growth factor that causes fibrosis. TGF-β1 can enhance the synthesis of extracellular matrix proteins such as collagen types I, III, and IV; laminin; and fibronectin.Citation7,Citation8 TGF-β1 also exerts its profibrotic activity by inhibition of fibrinolysis through plasminogen activator inhibitor-1Citation9 and maintenance of fibrosis through inhibition of matrix metalloproteinases.Citation10 It is now generally accepted that TGF-β1 is locally produced in the peritoneum and is likely to be the main growth factor for functional and structural changes of peritoneal membrane. TGF-β1 was induced in human peritoneal mesothelial cells (hPMCs) when exposed to various components of PDF.Citation11–13 In addition, TGF-β1 gene transferred to rat peritoneum induces prominent fibrosis in peritoneum.Citation14
CTGF is the primary mediator of the TGF-β1-induced fibrosis in various tissues. Zarrinkalam et al.Citation15 reported that CTGF can be produced in hPMCs in culture. Thereafter, several studies showed that both high glucose and TGF-β1 can increase the expression of CTGF as well as ECM in hPMCs.Citation16,Citation17 Recently, Mizutani et al.Citation18 suggest that high peritoneal transport state is associated with fibrosis and increased peritoneal CTGF expression in mesothelial cells. Moreover, CTGF knockdown were found to attenuate matrix protein production induced by TGF-β1 in cultured hPMCs.Citation19 Taken together, these data indicate that CTGF also play an important role in progressive peritoneal fibrosis.
Tanshinone IIA, a small compound extracted from Chinese medicine Danshen (the dried root or rhizome of Salvia miltiorrhiza Bunge) has been widely used in China and other countries for the treatment of cardiovascular and cerebrovascular diseases. Accumulating evidence suggests that Tanshinone IIA may suppress fibrosis and inflammation.Citation20–24 The present study was performed to investigate the effect of Tanshinone IIA on peritoneal fibrosis in a PD rat model treated with high-glucose-based PDF.
MATERIALS AND METHODS
Chemicals and Reagents
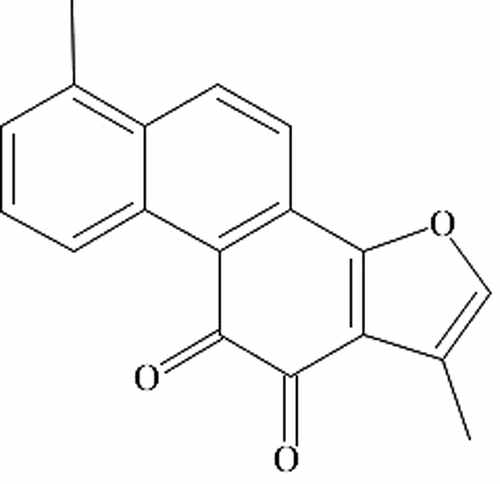
Tanshinone IIA was purchased from Jiangsu Carefree Group Co. (Nanjing, China). The structure of Tanshinone IIA is shown in . The 4.25% glucose-based PDF (Dianeal-N PD-4) was from Baxter Healthcare Co. Ltd. (Guangzhou, China). The polyclonal rabbit anti-rat TGF-β1 antibody, polyclonal goat anti-rat CTGF antibody, rabbit anti-actin antibody, and the enhanced chemiluminescence reagents were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Pv-6001 immunohistochemistry kit was purchased from Beijing Zhongsan Jinqiao Biotechnology (Beijing, China). Normal rabbit and goat serum were from GIBCO (Grand Island, NY, USA). TRIzol reagent and Moloney Murine Leukemia Virus Reverse Transcriptase was purchased from Invitrogen (Carlsbad, CA, USA). Taq DNA polymerase, dNTP, RNAsin, oligo(dT)15 primer, and oligonucleotides for TGF-β1, CTGF, and β-actin were from TaKaRa Biotech (Dalian, China). Recombinant rat TGF-β1, CTGF, and β-actin were from Wuhan EIAab Science Co. (Wuhan, China). Bovine serum albumin was purchased from Amresco Inc. (Solon, OH, USA).
Animals
Forty male Sprague-Dawley rats weighing 180–220 g were obtained from Nanjing University Model Animal Research Center (Nanjing, China). The animals were housed in the animal facility of Nanjing University Model Animal Research Center with free access to food and water. The Institutional Animal Care and Use Committee at Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, approved all the animal protocols. The experiments were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Experimental Procedures
The rats were randomly divided into four groups (n = 10 for each group). The first group (control) received daily intraperitoneal injection of 20 mL saline. The second group (PDF) received daily intraperitoneal injection of 20 mL 4.25% glucose-based PDF. And the other two groups received daily intraperitoneal injection of 20 mL 4.25% glucose-based PDF along with 50 mg/L (TL) or 100 mg/L (TH) Tanshinone IIA. After 8 weeks, we sacrificed all rats at 4 h after the last injection to obtain interior abdominal peritoneal wall and omentum for experiments.
Histological Analysis
Formalin-fixed, paraffin-embedded sections (2 µm) were stained with hematoxylin and eosin and Masson's trichrome for light microscopic observation. Measurement of the thickness of submesothelial compact zone (SCZ) was performed by image analysis at 200× original magnification using Image-Pro Plus 5.0 (Media Cybernetics). The five-point measurement method reported by Honda et al.Citation25 was used to calculate the average SCZ thickness.
Immunohistochemistry
Cryostat sections (2 µm thick) of interior abdominal peritoneal wall were processed using an indirect immunoperoxidase technique. The following primary antibodies were used: (1) polyclonal rabbit anti-rat TGF-β1 antibody; (2) polyclonal goat anti-rat CTGF antibody. Immunohistochemical detection for TGF-β1 and CTGF was performed using the pv-6001 immunohistochemistry kit and the images were analyzed using Image-Pro Plus 5.0. Negative controls were obtained by replacing the primary antibody with normal rabbit or goat serum.
Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from 100 mg omentum using TRIzol reagent according to the manufacturer's protocol. One microgram of total RNA was reverse transcribed to cDNA with reverse transcriptase at 37°C for 50 min and the resulting cDNA was subjected to polymerase chain reaction (PCR). The sequences of the PCR primers are as follows: TGF-β1, (forward) 5′-TGAGTGGCTGTCTTTTGACG-3′ and (reverse) 5′-TGGGACTGATCCCATTGATT-3′ (154 bp amplicon); CTGF, (forward) 5′-GCTAAGACCTGTGGAATGGGC-3′ and (reverse) 5′-CTCAAAGATGTCATTGCCCCC-3′ (383 bp); β-actin, (forward) 5′-CGCCAACCGCGAGAAGAT-3′) and (reverse) 5′-CGTCACCGGAGTCCATCA-3′ (168 bp). The cycling parameters were set as follows: 94°C for 30 s, 52°C for 30 s, 72°C for 30 s, with a final extension for 10 min at 72°C. The PCR products were subjected to agarose gel electrophoresis and the amount of TGF-β1 and CTGF was quantified using Image-Pro Plus 5.0. β-Actin was used as an internal control for normalization.
Western Blot Analysis
Frozen omentum was homogenized and the extracted protein (60–100 µg) was subjected to SDS-PAGE followed by electro-transfer onto polyvinylidene flouride (PVDF) membranes. The membranes were incubated with an antibody against TGF-β1, CTGF, or β-actin for 1 h. After three washes with Tris-Buffered NaCl Solution with Tween 20 (TBST), blots were incubated with horseradish peroxidase (HRP)-labeled secondary antibodies, washed and developed with enhanced chemiluminescence reagents. Recombinant rat TGF-β1 and CTGF proteins were used as positive controls. Bovine serum albumin was used as a negative control. The density of each protein band was quantified and normalized with β-actin.
Statistical Analysis
The results were expressed as mean ± SEM. Differences between groups were analyzed by one-way ANOVA followed by Dunnett's multiple comparison tests. Differences were considered to be statistically significant if p < 0.05. All analyses were performed using SPSS (Chicago, IL, USA).
RESULTS
Histological Changes in Parietal Peritoneum
Morphologic changes in parietal peritoneum were assessed by hematoxylin and eosin and Masson trichrome staining. At the end of 8 weeks, the peritoneal samples in PDF group showed marked thickening of the SCZ compared with control group (A and B). Tanshinone IIA evidently suppressed the thickness of the SCZ (C and D). Morphological evaluation revealed a significant dose-dependent inhibitory effect of Tanshinone IIA on PDF-induced SCZ thickening. Large areas of blue Masson trichrome stained matrix were observed in the peritoneum in PDF-treated rats, but not in control rats (p < 0.01, A and B). In contrast, the areas of blue Masson trichrome stained matrix in low-dose and high-dose Tanshinone IIA-treated rats were significantly reduced compared with non-treated PDF rats (p < 0.01, C and D). High-dose Tanshinone IIA is more effective in reducing the peritoneal matrix accumulation than low-dose Tanshinone IIA (p < 0.01).
Figure 2. Submesothelial compact zone (SCZ) thickness of parietal peritoneum (HE staining, 200× original magnification, scale bar represents 100 µm). The thickness of SCZ in PDF group rats (B) was significantly increased over that of control group rats (A). Treatment with Tanshinone IIA corrected the disturbances in morphology associated with PDF administration in a dose-dependent manner [(C) Low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. The mean SCZ thickness of each group of rats is presented in (E). Data are expressed as mean ± SEM.
Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.05 versus control, **p < 0.01 versus control, ***p < 0.05 versus PDF,****p < 0.01 versus PDF,*****p < 0.05 versus TL.
![Figure 2. Submesothelial compact zone (SCZ) thickness of parietal peritoneum (HE staining, 200× original magnification, scale bar represents 100 µm). The thickness of SCZ in PDF group rats (B) was significantly increased over that of control group rats (A). Treatment with Tanshinone IIA corrected the disturbances in morphology associated with PDF administration in a dose-dependent manner [(C) Low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. The mean SCZ thickness of each group of rats is presented in (E). Data are expressed as mean ± SEM.Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.05 versus control, **p < 0.01 versus control, ***p < 0.05 versus PDF,****p < 0.01 versus PDF,*****p < 0.05 versus TL.](/cms/asset/e20e0fd8-7a8d-4529-a132-0864d05f0a43/irnf_a_559681_f0002_b.jpg)
Figure 3. Submesothelial matrix of parietal peritoneum from rats (Masson staining, 200 × original magnification, scale bar represents 100 µm). Submesothelial matrix was faintly present in control group rats (A) and was prominent in PDF group rats (B). Treatment with Tanshinone IIA reduced the submesothelial matrix deposition in a dose-dependent manner [(C) low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. Submesothelial matrix is quantified in (E). Data are expressed as mean ± SEM.
Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.01 versus PDF, ***p < 0.01 versus TL.
![Figure 3. Submesothelial matrix of parietal peritoneum from rats (Masson staining, 200 × original magnification, scale bar represents 100 µm). Submesothelial matrix was faintly present in control group rats (A) and was prominent in PDF group rats (B). Treatment with Tanshinone IIA reduced the submesothelial matrix deposition in a dose-dependent manner [(C) low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. Submesothelial matrix is quantified in (E). Data are expressed as mean ± SEM.Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.01 versus PDF, ***p < 0.01 versus TL.](/cms/asset/6993e098-86a1-497c-a4bf-3a01b9b5e17f/irnf_a_559681_f0003_b.jpg)
Immunohistochemical Analysis of TGF-β1 and CTGF Expression in Parietal Peritoneum
Immunohistochemical analysis of TGF-β1 and CTGF expression showed some background staining in the parietal peritoneal tissues obtained from the control group (A and A). In contrast, a stronger, positive staining was found in PDF group (p < 0.01, B and 5B). Tanshinone IIA therapy significantly decreased the expression of TGF-β1 and CTGF in parietal peritoneum in a dose-dependent manner (C, D, 5C and D).
Figure 4. TGF-β1 expression in parietal peritoneum (400× original magnification). TGF-β1 was faintly present in control group rats (A) and was prominent in PDF group rats (B). Treatment with Tanshinone IIA reduced the TGF-β1 expression in a dose-dependent manner [(C) low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. (E) Negative control by replacing the primary antibody with normal rabbit serum. Expression of TGF-β1 is quantified in (F). Data are expressed as mean ± SEM.
Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.01 versus PDF, ***p < 0.01 versus TL.
![Figure 4. TGF-β1 expression in parietal peritoneum (400× original magnification). TGF-β1 was faintly present in control group rats (A) and was prominent in PDF group rats (B). Treatment with Tanshinone IIA reduced the TGF-β1 expression in a dose-dependent manner [(C) low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. (E) Negative control by replacing the primary antibody with normal rabbit serum. Expression of TGF-β1 is quantified in (F). Data are expressed as mean ± SEM.Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.01 versus PDF, ***p < 0.01 versus TL.](/cms/asset/58173bae-4dd2-4bc2-af2b-d112ab550d33/irnf_a_559681_f0004_b.jpg)
Figure 5. CTGF expression in parietal peritoneum (400× original magnification). CTGF was faintly present in control group rats (A) and was prominent in PDF group rats (B). Treatment with Tanshinone IIA reduced the CTGF expression in a dose-dependent manner [(C) low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. (E) Negative control by replacing the primary antibody with normal goat serum. Expression of CTGF is quantified in (F). Data are expressed as mean ± SEM.
Notes: TL, low-dose Tanshinone IIA-treated group; TH: high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.05 versus PDF, ***p < 0.05 versus TL.
![Figure 5. CTGF expression in parietal peritoneum (400× original magnification). CTGF was faintly present in control group rats (A) and was prominent in PDF group rats (B). Treatment with Tanshinone IIA reduced the CTGF expression in a dose-dependent manner [(C) low-dose Tanshinone IIA-treated group, (D) high-dose Tanshinone IIA-treated group]. (E) Negative control by replacing the primary antibody with normal goat serum. Expression of CTGF is quantified in (F). Data are expressed as mean ± SEM.Notes: TL, low-dose Tanshinone IIA-treated group; TH: high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.05 versus PDF, ***p < 0.05 versus TL.](/cms/asset/5d3e8081-ce69-4678-a26c-3a890d24d066/irnf_a_559681_f0005_b.jpg)
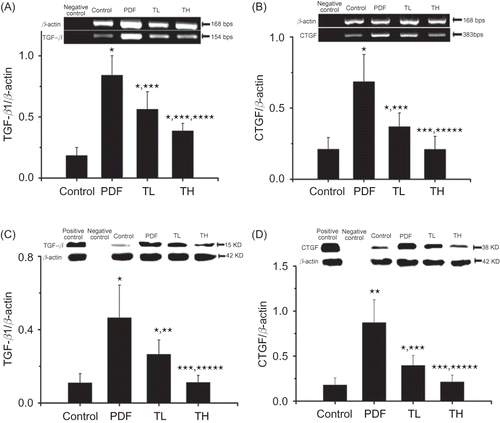
TGF-β1, CTGF mRNA, and Protein Expression in Omentum
Both mRNA and protein levels of TGF-β1 and CTGF significantly increased in the omentum of rats treated with PDF, compared with control rats (p < 0.01, A–D). Co-treatment of PDF with either low- or high-dose Tanshinone IIA markedly attenuated PDF-induced TGF-β1 or CTGF mRNA and protein expression (p < 0.01 or 0.05, respectively), although the high-dose Tanshinone IIA treatment showed a stronger inhibitory effect.
Figure 6. TGF-β1, CTGF mRNA, and protein expression in omentums. The expression of TGF-β1 and CTGF mRNA in the omentums was analyzed by RT-PCR (A: TGF-β1; B: CTGF). The expression of TGF-β1 and CTGF protein was analyzed by Western blotting (C: TGF-β1; D: CTGF). β-Actin was used as internal control (for RT-PCR) or loading control (Western blot) and all values were normalized to β-actin. The minor expression of TGF-β1 and CTGF were detected in the peritoneal tissues from control group rats, whereas TGF-β1 and CTGF expression was prominently increased in PDF-treated rats. Treatment with Tanshinone IIA reduced the TGF-β1 and CTGF expression in a dose-dependent manner. Data are expressed as mean ± SEM.
Notes: TL, low-dose Tanshinone IIA-treated group; TH, high-dose Tanshinone IIA-treated group; PDF, peritoneal dialysis fluid. *p < 0.01 versus control, **p < 0.05 versus PDF, ***p < 0.01 versus PDF, ****p < 0.05 versus TL, *****p < 0.01 versus TL.
DISCUSSION
In this study, we demonstrate that Tanshinone IIA treatment exerts beneficial effects in a rat model of peritoneal fibrosis induced by high glucose-based PDF. Our results show that Tanshinone IIA treatment reduced (1) the thickness of SCZ, (2) matrix accumulation in the peritoneum, (3) TGF-β1 and CTGF expression in parietal peritoneum, and (4) TGF-β1 and CTGF expression in omentum. These findings indicate that Tanshinone IIA inhibits PDF-induced peritoneal fibrosis through suppressing local TGF-β1 and CTGF expression levels in peritoneum.
Peritoneal fibrosis is one of the most common structural changes observed in patients undergoing long-term PD. The degree of fibrosis correlates with the duration of PD.Citation2,Citation3,Citation26,Citation27 For a long time, fibrosis has been considered as the main cause of the progressive functional decline in the peritoneum and ultrafiltration failure. In our animal model of PD in which the peritoneum was exposed to PDF for over 8 weeks, we observed changes similar to those seen in long-term PD patients with increased SCZ thickening and matrix accumulation in the peritoneum, which coincide with the changes in peritoneum induced by PDF reported in previous studies.Citation28
Our findings showing that TGF-β1 expression in parietal peritoneum and omentum increased in rats treated with PDF indicate that TGF-β1 plays a key role in peritoneal fibrosis induced by PDF. Although CTGF has been known as a growth factor important for extracellular matrix production and other profibrotic activities mediated by TGF-β1, evidence suggesting a role for CTGF in peritoneal fibrosis mainly comes from in vitro cell culture models, and few studies have focused on its role in vivo. Our data demonstrate that CTGF expression in parietal peritoneum and omentum increases in rats treated with PDF, suggesting that CTGF is involved in peritoneal fibrosis induced by PDF in vivo.
Several recent studies showed that additives into conventional PDF may improve the bio-compatibility of conventional PDF or inhibit the process of peritoneal fibrosis. Matsuo et al. examined the effects of hepatocyte growth factor (HGF) on peritoneal fibrosis induced by high concentrations of glucose, and observed that HGF rescues the growth inhibition of mesothelial cells cultured in high concentrations of glucose. They concluded that exogenous HGF could possibly prevent peritoneal fibrosis.Citation29 In vivo experiments on rats undergoing PD also showed that pyridoxal 5′-phosphate markedly inhibited the peritoneal thickening induced by PDF as well as decreased the TGF-β1 and type I collagen expression in the visceral peritoneum.Citation30 Similarly, the addition of aminoguanidines to PDF has also been found to inhibit the fibrosis in animal models.Citation31,Citation32
Recently, several studies have shown that Tanshinone IIA possesses remarkable anti-fibrosis properties.Citation20–24 For example, Tanshinone IIA can attenuate cardiac fibrosis and modulate collagen metabolism in rats with renovascular hypertension.Citation33 It also prevents hepatic and renal fibrosis.Citation34,Citation35 To our knowledge, our study is the first to show the beneficial effects of Tanshinone IIA on preventing peritoneal fibrosis when it is combined with conventional PDF. The co-treatment of PDF with either low- or high-dose Tanshinone IIA significantly inhibits PDF-induced increase of TGF-β1 and CTGF expression in both parietal peritoneum and omentum. This result indicates that Tanshinone IIA is an effective agent for preventing peritoneal fibrosis.
Tanshinone IIA is a diterpene that is thought to be a major bioactive molecule in Danshen, a dietary supplement derived from the root of S. miltiorrhiza, which is widely used in China and other Asian countries. The toxicity of Tanshinone IIA in vivo and in vitro has been well studied.Citation36 Data obtained from mice model show that the LD50 of Tanshinone IIA is 25.807 g/kg, which is equivalent to approximately 4000 folds the intended clinical human oral dosage. Moreover, an oral dose of 2500 mg/kg Danshen extract (400 folds the human oral dosage) for 90 days was found to be nontoxic to rats.Citation37 Data from in vitro studies also indicate that Tanshinone IIA does not inhibit the viability of rat cardiac cells.Citation23 Consistently, we found that Tanshinone IIA had no effect on cell viability in cultured hPMCs. Neither does it affect the secretion of TGF-β1 or CTGF (data not shown). Taken together, these data suggest that it is safe to use Tanshinone IIA in clinical practice to prevent peritoneal fibrosis in patients undergoing long-term PD.
There are several limitations in this study. First, it is well known that both bio-incompatible PDF and uremic status are the main factors that lead to structural and functional changes in peritoneum. A non-uremic animal model used in this study may lead to an underestimation of the PDF-induced injury under uremic conditions. Second, in this study, we did not have a matching control group of rats that were treated with Tanshinone alone. Despite the fact that Tanshinone IIA had no effect on cell viability or TGF-β1 and CTGF secretion in cultured hPMCs, the in vivo effect of the drug alone on the morphology and the expression of these factors in peritoneum are worth further study.
In conclusion, Tanshinone IIA significantly inhibits the PDF-induced upregulation of TGF-β1 and CTGF in peritoneum and thus may potentially be used in clinic to prevent peritoneal fibrosis during long-term dialysis. Further studies are necessary to elucidate the molecular mechanism by which Tanshinone IIA inhibits the expression of TGF-β1 and CTGF.
ACKNOWLEDGMENT
This study was supported by the Key Science and Technology Development Program of Nanjing City (ZKX08026).
Declaration of interest: The authors report no conflicts of interest. The author alone is responsible for the content and writing of the paper.
REFERENCES
- Di Paolo N, Sacchi G. Atlas of peritoneal histology. Perit Dial Int. 2000;20(Suppl. 3):S5–S96.
- Krediet RT. The peritoneal membrane in chronic peritoneal dialysis. Kidney Int. 1999;55:341–356.
- Williams JD, Craig KJ, Topley N, . Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479.
- Ito T, Yorioka N. Peritoneal damage by peritoneal dialysis solutions. Clin Exp Nephrol. 2008;12:243–249.
- Gonzalez-Mateo GT, Loureiro J, Jimenez-Hefferman JA, . Chronic exposure of mouse peritoneum to peritoneal dialysis fluid: Structural and functional alterations of the peritoneal membrane. Perit Dial Int. 2009;29:227–230.
- Ha H, Cha MK, Choi HN, Lee HB. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit Dial Int. 2002;22:171–177.
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292.
- Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508.
- Rougier JP, Guia S, Hagege J, Nguyen G, Ronco PM. PAI-1 secretion and matrix deposition in human peritoneal mesothelial cell cultures: Transcriptional regulation by TGF-beta 1. Kidney Int. 1998;54:87–98.
- Martin J, Yung S, Robson RL, Steadman R, Davies M. Production and regulation of matrix metalloproteinases and their inhibitors by human peritoneal mesothelial cells. Perit Dial Int. 2000;20:524–533.
- Yung S, Chen XR, Tsang RC, Zhang Q, Chan TM. Reduction of perlecan synthesis and induction of TGF-beta1 in human peritoneal mesothelial cells due to high dialysate glucose concentration: Implication in peritoneal dialysis. J Am Soc Nephrol. 2004;15:1178–1188.
- Naiki Y, Maeda Y, Matsuo K, . Involvement of TGF-beta signal for peritoneal sclerosing in continuous ambulatory peritoneal dialysis. J Nephrol. 2003;16:95–102.
- Ha H, Yu MR, Lee HB. High glucose-induced PKC activation mediates TGF-beta 1 and fibronectin synthesis by peritoneal mesothelial cells. Kidney Int. 2001;59:463–470.
- Margetts PJ, Kolb M, Galt T, Hoff CM, Shockley TR, Gauldie J. Gene transfer of transforming growth factor-beta1 to the rat peritoneum: Effects on membrane function. J Am Soc Nephrol. 2001;12:2029–2039.
- Zarrinkalam KH, Stanley JM, Gray J, Oliver N, Faull RJ. Connective tissue growth factor and its regulation in the peritoneal cavity of peritoneal dialysis patients. Kidney Int. 2003;64:331–338.
- Sakamoto N, Sugimura K, Kawashima H, . Influence of glucose and inflammatory cytokines on TGF-beta1 and CTGF mRNA expressions in human peritoneal mesothelial cells. Int J Mol Med. 2005;15:907–911.
- Szeto CC, Lai KB, Chow KM, Szeto CY, Wong TY, Li PK. Differential effects of transforming growth factor-beta on the synthesis of connective tissue growth factor and vascular endothelial growth factor by peritoneal mesothelial cell. Nephron Exp Nephrol. 2005;99:e95–e104.
- Mizutani M, Ito Y, Mizuno M, . Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am J Physiol Renal Physiol. 2010;298:F721–F733.
- Xiao L, Sun L, Liu FY, Peng YM, Duan SB. Connective tissue growth factor knockdown attenuated matrix protein production and vascular endothelial growth factor expression induced by transforming growth factor-beta1 in cultured human peritoneal mesothelial cells. Ther Apher Dial. 2010;14:27–34.
- Liu T, Jin H, Sun QR, Xu JH, Hu HT. The neuroprotective effects of tanshinone IIA on beta-amyloid-induced toxicity in rat cortical neurons. Neuropharmacology. 2010;59:595–604.
- Yang L, Zou XJ, Gao X, . Sodium tanshinone IIA sulfonate attenuates angiotensin II-induced collagen type I expression in cardiac fibroblasts in vitro. Exp Mol Med. 2009;41:508–516.
- Li YI, Elmer G, Leboeuf RC. Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase. Life Sci. 2008;83:557–562.
- Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–221.
- Tang F, Wu X, Wang T, . Tanshinone IIA attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vascul Pharmacol. 2007;46:427–438.
- Honda K, Hamada C, Nakayama M, . Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol. 2008;3:720–728.
- Selgas R, Fernandez-Reyes MJ, Bosque E, . Functional longevity of the human peritoneum: How long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am J Kidney Dis. 1994;23:64–73.
- Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998;54:2207–2217.
- Kihm LP, Wibisono D, Muller-Krebs S, . Rage expression in the human peritoneal membrane. Nephrol Dial Transplant. 2008;23:3302–3306.
- Matsuo K, Maeda Y, Naiki Y, . Possible effects of hepatocyte growth factor for the prevention of peritoneal fibrosis. Nephron Exp Nephrol. 2005;99:e87–e94.
- Nakamura S, Niwa T. Pyridoxal phosphate and hepatocyte growth factor prevent dialysate-induced peritoneal damage. J Am Soc Nephrol. 2005;16:144–150.
- Zareie M, Tangelder GJ, Ter Wee PM, . Beneficial effects of aminoguanidine on peritoneal microcirculation and tissue remodelling in a rat model of PD. Nephrol Dial Transplant. 2005;20:2783–2792.
- Lee EA, Oh JH, Lee HA, . Structural and functional alterations of the peritoneum after prolonged exposure to dialysis solutions: Role of aminoguanidine. Perit Dial Int. 2001;21:245–253.
- Fang J, Xu SW, Wang P, . Tanshinone IIA attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine. 2010;18:58–64.
- Sun RF, Liu LX, Zhang HY. Effect of Tanshinone II on hepatic fibrosis in mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:1012–1017.
- Tang J, Zhan C, Zhou J. Effects of Tanshinone IIA on transforming growth factor beta1-Smads signal pathway in renal interstitial fibroblasts of rats. J Huazhong Univ Sci Technol Med Sci. 2008;28:539–542.
- Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–148.
- Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359.