Abstract
Objective: Uremic pruritus (UP) remains a frequent problem in hemodialysis (HD) patients and is related to mortality. Poor glycemic control, as evaluated by hemoglobin A1c (HbA1c), is also associated with morbidity and mortality in HD patients. In this study, we investigated the relationship between UP and HbA1c in HD patients. Methods: Sociodemographic, clinical, and laboratory variables, depressive symptoms, and health-related quality of life were assessed. Severity of UP was evaluated by visual analogue scale (VAS). The scale consisted of a 10 cm horizontal line marked from 0 (denoting no itch) to 10 (denoting worst possible imaginable itch). Results: Totally, 75 patients (male/female, 41/34; diabetic/nondiabetic, 29/46; age, 51.9 ± 13.5 years) were included. The VAS pruritus score was higher in diabetic patients compared with nondiabetic patients (4.7 ± 2.8 vs. 3.0 ± 1.0, p : 0.015). In diabetics, VAS pruritus score was independently related with calcium–phosphorus product (β : +0.637, p < 0.0001), intact parathyroid hormone (β : +0.343, p : 0.017), HbA1c (β : +0.310, p : 0.027), and Beck depression score (β : +0.474, p : 0.002). In nondiabetics, VAS pruritus score was independently related with calcium–phosphorus product (β : +0.486, p : 0.004), intact parathyroid hormone (β : +0.302, p : 0.041), and HbA1c (β : +0.341, p : 0.033). In the whole patient group, VAS pruritus score was independently related with calcium–phosphorus product (β : +0.372, p : 0.001), intact parathyroid hormone (β : +0.241, p : 0.008), HbA1c (β : +0.227, p : 0.031), and Beck depression score (β : +0.298, p : 0.003). Conclusions: In both diabetic and nondiabetic patients, HbA1c is closely related with pruritus in HD patients.
INTRODUCTION
Uremic pruritus (UP), defined as an uncomfortable sensation that elicits the desire to scratch, has been well recognized as a common complication and related to mortality in patients with chronic renal failure.Citation1–4 The pathogenic basis of UP in chronic renal failure has not been resolved. However, various theories exist including secondary hyperparathyroidism, divalent-ion abnormalities, metabolic abnormalities, histamine, allergic sensitization, proliferation of skin mast cells, hypervitaminosis A, xerosis, opioid system involvement, cytokines, or the combination of these.Citation5,Citation6 Besides these factors, other less well-known factors were also thought to be related with UP. One of these factors is the presence of neuropathy. Indeed, it has been suggested that the activity of the nervous system plays an important role in the mechanism of UP and, in most of the renal failure patients, the skin innervation is nonspecifically altered, possibly as a consequence of neuropathy.Citation6–9
Glycated hemoglobin A1c (HbA1c) is formed by the binding of glucose to the N-terminal amino groups of the hemoglobin, which occurs continuously during the life of red blood cells in a nonenzymatic process.Citation10 There are reports showing that HbA1c levels are related to mortality in hemodialysis (HD) patients.Citation11,Citation12 Apart from mortality, total hyperglycemia exposure, as determined by HbA1c, has been shown to be an important risk factor and independently associated with the occurrence of diabetic polyneuropathy.Citation13–15 Patients with both uremia and diabetes mellitus frequently develop polyneuropathy.Citation16 Thus, based on the above-mentioned findings, it can be concluded that (1) both UP and HbA1c are related with mortality in HD patients, (2) there are studies showing that higher HbA1c is a risk factor for development of neuropathy in general population, (3) UP might be related with neuropathy, and (4) patients with uremia, as well as patients with diabetes mellitus, develop polyneuropathy. Bearing these issues in mind, we hypothesized that common mechanisms regarding UP and HbA1c may be operating in the increased mortality in HD patients. Therefore, we conducted this study to determine whether HbA1c levels were independently related with UP.
MATERIALS AND METHODS
The subjects for our cross-sectional investigation were HD patients attending the HD unit in Zonguldak Atatürk State Hospital. This study was in accordance with the declaration of Helsinki and written informed consent was obtained from all patients. We recorded the sociodemographic and clinical characteristics of the patients. None of the patients were receiving any systemic drugs for pruritus and neuropathy, including antihistamines and gabapentin. None of the patients had concomitant dermatological, liver, or metabolic diseases associated with pruritus. Body mass index was calculated as the ratio of dry weight in kilograms (end-dialysis weight) to height squared (in square meters). The patients were asked to record the severity of their pruritus on a visual analogue scale (VAS). The VAS pruritus scale consisted of a 10 cm horizontal line marked from 0 (denoting no itch) to 10 (denoting worst possible imaginable itch). The depressive symptoms and quality of life were evaluated by the Beck Depression Inventory (BDI) and by SF-36, respectively.Citation17,Citation18
The dialysis prescription in our study included 4–5 h of HD, with blood-flow rates of 300–400 mL/min, using standard bicarbonate dialysis solution (containing glucose for diabetic patients) and hollow fiber dialyzers with surface area of 1.3–2 m2, as appropriate (Kwasumi Laboratories, Inc., Tokyo, Japan). The routine laboratory parameters were measured before the beginning of HD session. The laboratory parameters including serum hemoglobin, albumin, high-sensitive C-reactive protein (Hs-CRP), predialysis calcium and phosphorus, predialysis blood urea nitrogen and creatinine, total cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), high-density lipoprotein cholesterol (HDL-cholesterol), triglycerides, and HbA1c were measured before beginning the dialysis session. Postdialysis serum urea nitrogen concentrations, used to calculate the urea reduction ratio, were also measured.
Statistics
Statistical analysis was performed using SPSS 15.0 (SPSS Inc., Evanston, IL, USA). Results were considered statistically significant if two-tailed p-value was less than 0.05. Data are shown as mean ± standard deviation, median, and percentage. Data were checked for normality. Comparisons between two groups were assessed by means of t-test for normally distributed continuous variables and Mann–Whitney U test for non-normally distributed continuous variables. Pearson's correlation coefficient, r, was used for correlations. For the analysis of categorical variables, we used the chi-square test. Linear regression analysis was performed to analyze the independent factors related with VAS pruritus score. Variables tested for significance included age, HD duration, gender, single pool Kt/V (spKt/V), hemoglobin, calcium–phosphorus product, intact parathyroid hormone, alanine aminotransferase, aspartate aminotransferase, anti-hepatitis C virus anticore (anti-HCV), Hs-CRP, HbA1c, Beck depression score, and physical and mental component summary scores.
RESULTS
In total, 75 patients (male/female, 41/34; diabetic/nondiabetic, 29/46; age, 51.9 ± 13.5 years) were included. The etiologies for the end stage renal disease (ESRD) were as follows: diabetes in 29 patients, hypertension in 15 patients, glomerulonephritis in 10 patients, vesicourethral reflux and pyelonephritis in 4 patients, amyloidosis in 3 patients, nephrolithiasis in 3 patients, polycystic kidney disease in 3 patients, contrast nephropathy in 1 patient, Alport syndrome in 1 patient, and ischemic nephropathy in 1 patient. The etiology was unknown in five patients. In total four patients had anti-HCV. The baseline demographic characteristics of all patients in general and by presence of diabetes mellitus are shown in and the laboratory parameters are shown in .
Table 1. The baseline demographic characteristics of the patients by presence of diabetes mellitus and in general
Table 2. The laboratory parameters of the patients by presence of diabetes mellitus and in general
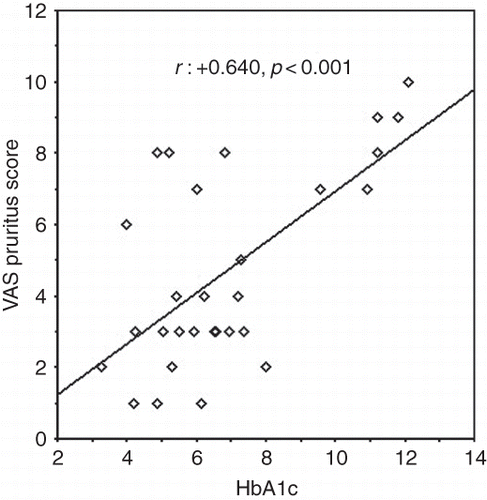
In diabetic patients, VAS pruritus score was correlated with the following parameters: Beck depression score (r : +0.803, p < 0.0001), blood glucose (r : +0.271, p : 0.019), serum calcium (r : +0.714, p < 0.0001), serum phosphorus (r : +0.700, p < 0.0001), calcium–phosphorus product (r : +0.781, p < 0.0001), intact parathyroid hormone (r : +0.612, p < 0.0001), and HbA1c (r : +0.640, p < 0.0001; ).
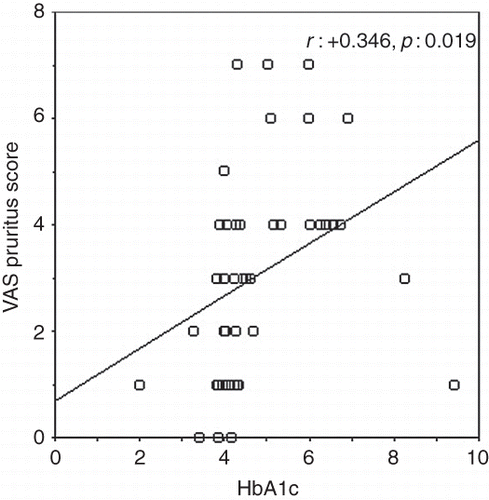
In nondiabetic patients, VAS pruritus score was correlated with the following parameters: serum phosphorus (r : +0.379, p : 0.009), calcium–phosphorus product (r : +0.413, p : 0.004), intact parathyroid hormone (r : +0.324, p : 0.028), and HbA1c (r : +0.346, p : 0.019; ).
Figure 2. Correlation graph between VAS pruritus score and HbA1c in nondiabetic hemodialysis patients.
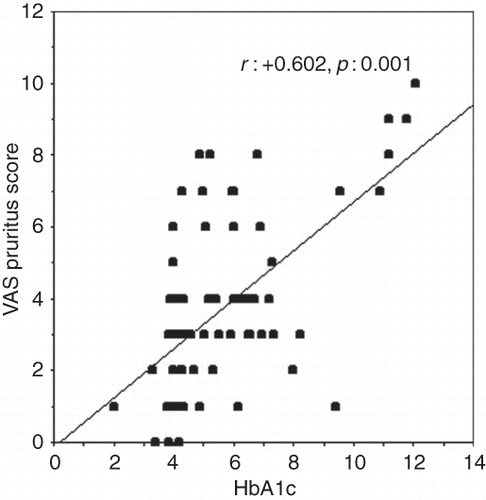
In the whole patient group, VAS pruritus score was correlated with the following parameters: Beck depression score (r : +0.583, p < 0.0001), blood glucose (r : +0.271, p = 0.019), serum calcium (r : +0.534, p < 0.0001), serum phosphorus (r : +0.521, p < 0.0001), calcium–phosphorus product (r : +0.609, p < 0.0001), intact parathyroid hormone (r : +0.422, p < 0.0001), and HbA1c (r : +0.602, p < 0.0001; ).
Of note none of the other laboratory parameters quality-of-life parameters were related VAS pruritus score (data not shown).
In diabetics, VAS pruritus score was independently related with calcium–phosphorus product, intact parathyroid hormone, HbA1c, and Beck depression score ().
Table 3. The multiple linear analyses of factors independently associated with visual analogue scale pruritus score of the patients by presence of diabetes mellitus and in general
In nondiabetics, VAS pruritus score was independently related with calcium–phosphorus product, intact parathyroid hormone, and HbA1c ().
In the whole patient group, VAS pruritus score was independently related with calcium–phosphorus product, intact parathyroid hormone, HbA1c, and Beck depression score ().
DISCUSSION
It is demonstrated that UP is related to mortality in dialysis patients.Citation3,Citation4 Although the underlying pathogenic mechanisms are not fully known, based on several observations and results from various trials on UP, there is increasing evidence that UP is a systemic rather than an isolated skin disease and the discussion has focused on metabolic abnormalities, such as secondary hyperparathyroidism, precipitated calcium phosphate crystals, and histamine secretion by mast cells, as underlying causes of UP.Citation4,Citation19
There is an unproven idea that diabetic patients complain of pruritus more frequently than the general population, and many clinicians may have an impression that pruritus is more frequent in diabetic patients than in nondiabetic individuals. However, there is still little epidemiological evidence of pruritus with diabetes. In a very recent study, it was demonstrated that numbness and dysesthesia in toes and soles and pain in feet were all more frequent in diabetic patients when compared with nondiabetic patients. Additionally, pruritus was also more common in diabetic patients when compared with nondiabetic patients. The prevalence of pruritus in diabetic subjects was significantly higher than that in nondiabetic subjects (26.3% vs. 14.6%, p < 0.001). The authors suggested that truncal pruritus of unknown origin was suspected of being a complication of diabetes, and diabetic polyneuropathy was suspected of being a possible origin of truncal pruritus of unknown origin.Citation20 To the best of our knowledge, in HD patients there is no study that compared the pruritus prevalence of diabetic and nondiabetic patients. As a novel finding, in this study we found that diabetic HD patients experienced more severe pruritus, as evaluated by VAS pruritus score, than nondiabetic HD patients and, interestingly, in both diabetic and nondiabetic patients, UP was independently related with HbA1c. We do not know the exact mechanisms regarding this relationship; however, we speculate that neuropathy may be one of the responsible factors for the development of pruritus in our HD patients. It is well known that patients with uremia, as well as patients with diabetes mellitus, develop polyneuropathy,Citation16 and peripheral nervous system of diabetic patients becomes more affected when these patients develop uremia.Citation21 There are very few studies directly comparing the neuropathy in end-stage renal disease patients with and without diabetes. In one study, it was shown that polyneuropathy was significantly worse in diabetic than in nondiabetic patients on peritoneal dialysis. Additionally, significant correlations were found between the analyzed neurophysiological parameters and duration of dialysis, duration of diabetes, glucose concentration, HbA1c, and dialysis adequacy.Citation16 Thus, diabetes per se may have a direct and an additional effect on the development of neuropathy in dialysis patients, but the exact mechanisms remain to be determined. Thus, the development of neuropathy (whether uremic, diabetic, or both) may have played a significant role in the development of pruritus in our HD patients. Additionally, in diabetic HD patients, possible diabetic neuropathy may have further increased the severity of pruritus. Previous studies have provided useful insights into the role of hyperglycemia and relation of HbA1c with diabetic polyneuropathy.Citation13,Citation22–26 These findings are not surprising because it is a well-known notion that tight glycemic control, as measured using HbA1c levels, decreases the risk of developing retinopathy, nephropathy, and neuropathy in the general population.Citation12 Although we did not perform any test to detect neuropathy, all the above evidence might explain that our diabetic HD patients who experienced more severe pruritus than nondiabetics may have had additional diabetic neuropathy as a result of poor glycemic control, as demonstrated by high HbA1c levels.
Another interesting and unexpected finding in this study was that in nondiabetic HD patients, HbA1c levels were also independently related with UP. This is a surprising and difficult-to-explain finding for us. Again, neuropathy of uremic origin may be the underlying culprit. As the itching sensation is thought to be transmitted by small, unmyelinated sensory c-fibers,Citation27 pruritus may reflect some abnormality of the peripheral nerve.Citation20 Indeed, it was previously hypothesized that neuropathy seen in dialysis patients might reflect a dysfunction of the peripheral nervous system.Citation16,Citation28 Additionally, as uremia progresses, peripheral nervous system becomes more affected, especially the sensory parts.Citation21 On the other hand, reports showing the efficacy of lidocaine,Citation29 capsaicin, Citation30–32 and gabapentin Citation33,Citation34 in controlling UP are in favor of a relationship between neuropathy and pruritus in HD patients. Thus, we can only hypothesize that even in nondiabetic patients, disturbed glucose metabolism as demonstrated by higher HbA1c levels, at least by inducing permissive role for the development of neuropathy, may result in increased sensation of itching.
In our study, we found an independent relationship between calcium–phosphorus product and intact parathyroid hormone with UP. Our findings are not novel as it was demonstrated that the risk of UP increased in patients with severely elevated serum calcium and phosphorus levels and calcium–phosphorus product.Citation4,Citation30,Citation35–39 Lastly, the relationship between depression and UP is not a novel finding as well, being reported previously in other studies.Citation39,Citation40
In conclusion, the two parameters related to mortality in HD patients, namely pruritus and HbA1c, are independently related with each other. Our preliminary study shows that studies are needed to highlight the mechanisms defining the relationship between HbA1c and UP.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Ponticelli C, Bencini PL. Pruritus in dialysis patients: A neglected problem. Nephrol Dial Transplant. 1995;10:2174–2176.
- Schwartz IF, Iaina A. Uraemic pruritus. Nephrol Dial Transplant. 1999;14:834–839.
- Carmichael AJ, McHugh MI, Martin AM. Renal itch as an indicator of poor outcome. Lancet. 1991;337:1225–1226.
- Narita I, Alchi B, Omori K, Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69:1626–1632.
- Narita I, Iguchi S, Omori K, Gejyo F. Uremic pruritus in chronic hemodialysis patients. J Nephrol. 2008;21:161–165.
- Akhyani M, Ganji MR, Samadi N, Khamesan B, Daneshpazhooh M. Pruritus in hemodialysis patients. BMC Dermatol. 2005;24(5):7.
- Zakrzewska-Pniewska B, Jedras M. Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R–R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol Clin. 2001;31:181–193.
- Johansson O, Hilliges M, Stahle-Backdahl M. Intraepidermal neuron-specific enolase (NSE)-immunoreactive nerve fibres: Evidence for sprouting in uremic patients on maintenance hemodialysis. Neurosci Lett. 1989;99:281–286.
- Fantini F, Baraldi A, Sevignani C, Spattini A, Pincelli C, Giannetti A. Cutaneous innervation in chronic renal failure patients. An immunohistochemical study. Acta Derm Venereol. 1992;72:102–105.
- Nakao T, Matsumoto H, Okada T, Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern Med. 1998;37:826–830.
- Wu MS, Yu CC, Yang CW, Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance hemodialysis. Nephrol Dial Transplant. 1997;12:2105–2110.
- Kalantar-Zadeh K, Kopple JD, Regidor DL, A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–1055.
- Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ, III, O'Brien PC. Risk factors for severity of diabetic polyneuropathy: Intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care. 1999;22:1479–1486.
- Bognetti E, Calori G, Meschi F, Macellaro P, Bonfanti R, Chiumello G. Prevalence and correlations of early microvascular complications in young type I diabetic patients: Role of puberty. J Pediatr Endocrinol Metab. 1997;10:587–592.
- Gulcelik NE, Karakaya J, Gedik A, Usman A, Gurlek A. Serum vaspin levels in type 2 diabetic women in relation to microvascular complications. Eur J Endocrinol. 2009; 160:65–70.
- Jovanovic DB, Matanovic DD, Simic-Ogrizovic SP, Stosovic MD, Bontic AC, Nesic VD. Polyneuropathy in diabetic and nondiabetic patients on CAPD: Is there an association with HRQOL? Perit Dial Int. 2009;29:102–107.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571.
- Ware JE, Jr., Sherbourne CD. The MOS 36 item short form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483.
- Kimmel M, Alscher DM, Dunst R, The role of micro-inflammation in the pathogenesis of uraemic pruritus in hemodialysis patients. Nephrol Dial Transplant. 2006;21:749–755.
- Yamaoka H, Sasaki H, Yamasaki H, Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150–155.
- Hassan K, Simri W, Rubenchik I, Effects of erythropoietin therapy on polyneuropathy in predialytic patients. J Nephrol. 2003;16:121–125.
- Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309.
- Gregersen G. Diabetic neuropathy: Influence of age, sex, metabolic control, and duration of diabetes on motor conduction velocity. Neurology. 1967;17:972–980.
- Graf RJ, Halter JB, Halar E, Porte D, Jr. Nerve conduction abnormalities in untreated maturity-onset diabetes: Relation to levels of fasting plasma glucose and glycosylated hemoglobin. Ann Intern Med. 1979;90:298–303.
- Porte D, Jr, Graf RJ, Halter JB, Pfeifer MA, Halar E. Diabetic neuropathy and plasma glucose control. Am J Med. 1981;70:195–200.
- Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF. Risk factors for distal symmetric neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1994;17:1172–1177.
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008.
- Murphy M, Carmichael AJ. Renal itch. Clin Exp Dermatol. 2000;25:103–106.
- Tapia L, Cheigh JS, David DS, Pruritus in dialysis patients treated with parenteral lidocaine. N Engl J Med. 1977;296:261–262.
- Cho YL, Liu HN, Huang TP, Tarng DC. Uremic pruritus: Roles of parathyroid hormone and substance P. J Am Acad Dermatol. 1997;36:538–543.
- Weisshaar E, Dunker N, Gollnick H. Topical capsaicin therapy in humans with hemodialysis-related pruritus. Neurosci Lett. 2003;345:192–194.
- Tarng DC, Cho YL, Liu HN, Huang TP. Hemodialysis-related pruritus: A double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron. 1996;72:617–622.
- Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in hemodialysis patients: A randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19:3137–3139.
- Manenti L, Vaglio A, Costantino E, Gabapentin in the treatment of uremic itch: An index case and a pilot evaluation. J Nephrol. 2005;18:86–91.
- Noordzij M, Boeschoten EW, Bos WJ, Disturbed mineral metabolism is associated with muscle and skin complaints in a prospective cohort of dialysis patients. Nephrol Dial Transplant. 2007;22:2944–2949.
- Massry SG, Popovtzer MM, Coburn JW, Makoff DL, Maxwell MH, Kleeman CR. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N Engl J Med. 1968;279:697–700.
- Urbonas A, Schwartz RA, Szepietowski JC. Uremic pruritus-an update. Am J Nephrol. 2001;21:343–350.
- Momose A, Kudo S, Sato M, Calcium ions are abnormally distributed in the skin of hemodialysis patients with uraemic pruritus. Nephrol Dial Transplant. 2004;19:2061–2066.
- Wikström B. Itchy skin – a clinical problem for hemodialysis patients. Nephrol Dial Transplant. 2007;22(Suppl. 5):v3–v7.
- Pisoni RL, Wikström B, Elder SJ, Pruritus in hemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21:3495–3505.