Abstract
Background: Patients with decreased renal function are characterized by high cardiovascular morbidity and mortality due to complications of premature atherosclerosis. Placental growth factor (PlGF) is a proatherogenic cytokine and new biomarker of cardiovascular events. The aim of this study was to determine PlGF levels and describe their relationship to renal function and risk factors of atherogenesis in patients with decreased renal function. Methods: The study group consisted of 114 subjects: 45 patients with various degrees of decreased renal function (CHRI), 31 long-term hemodialysis (HD) patients, and 38 age-matched healthy control subjects. PlGF was assessed immunochemically (enzyme-linked immunosorbent assay) and routine biochemical parameters were measured using standard laboratory methods. Results: PlGF levels were significantly increased in CHRI and HD patients compared to controls (10.5 ± 3.3 pg/mL in CHRI patients and 11.5 ± 3.4 pg/mL HD patients vs. 8.1 ± 1.8 pg/mL in controls, both p < 0.0001). In CHRI patients, PlGF was detectable in the urine, and its urine concentration correlated with its serum levels. In HD patients, PlGF correlated with low-density lipoproteins (r = 0.36, p < 0.05), but was not related to C-reactive protein levels. Higher levels of PlGF were found in CHRI patients with cardiovascular disease, compared with those free of such complication. Conclusions: PlGF levels are increased in patients with decreased kidney function. PlGF is detectable in the urine, and serum and urine levels of PlGF are significantly interrelated. It is higher in CHRI patients with cardiovascular disease. Further studies are required to demonstrate the usefulness and significance of PlGF in patients with chronic kidney disease.
INTRODUCTION
Patients with chronic kidney disease are characterized by high cardiovascular morbidity and mortality due to complications of premature atherosclerosis such as coronary heart disease,Citation1–3 and cardiovascular complications are the major cause of death.Citation4 Atherosclerosis is now assumed to be a complex endothelial dysfunction induced by elevated and modified low-density lipoproteins (LDL), free radicals, infectious microorganisms, shear stress, hypertension, toxins after smoking, or a combination of these and other factors leading to a compensatory inflammatory response.Citation5 Because accelerated atherosclerosis is one of the consequential complications of chronic kidney disease and hemodialysis,Citation6 placental growth factor (PlGF) might be an early marker of vascular inflammation possibly related to cardiovascular outcome.
PlGF is a member of the vascular endothelial growth factor (VEGF) family. PlGF is a 50-kDa heterodimer consisting of 149 amino acids and has high homology with VEGF.Citation7 PlGF exists in at least four alternatively spliced forms: PlGF-1, PlGF-2, PlGF-3, and PlGF-4.Citation8–11 Notable differences between these forms include the insertion of a heparin-binding domain in PlGF-2 and PlGF-4 that might result in increased association with cell membrane or altered affinities for PlGF receptors.Citation12 As the name implies, PlGF was first identified in human placenta and, indeed, is expressed prominently in placenta under normal conditions.Citation7–9,Citation13 Other tissues expressing PlGF include the heart, thyroid gland, lung, and skeletal muscle.Citation14 Increased PlGF levels have been described in several conditions including cancer,Citation15–17 cutaneous wound and bone fracture healing,Citation18–22 and sickle cell disease.Citation22
PlGF was recently shown to be upregulated in early and advanced atherosclerotic lesions.Citation23 PlGF stimulates vascular smooth muscle growth, recruits macrophages into atherosclerotic lesions, upregulates production of tumor necrosis factor α and monocyte chemotactic protein-1 by macrophages, and stimulates pathological angiogenesis.Citation8,Citation23 Inhibition of PlGF effects by blocking of its receptor, Fms-like tyrosine kinase, in an animal model suppressed both atherosclerotic plaque growth and vulnerability through inhibition of inflammatory cell infiltration.Citation23 These data suggest that PlGF may act as a primary instigator of atherosclerotic lesions.
The aim of our study was to determine serum PlGF levels in patients with decreased kidney function including hemodialysis patients, to investigate the association between PlGF and serum lipids as markers of dyslipidemia and C-reactive protein (CRP) as an inflammatory marker.
METHODS
Patients and Controls
PlGF was studied in patients with chronic kidney diseases and various degrees of decreased renal function (CHRI), hemodialysis (HD) patients, and age-matched healthy controls. Detailed characteristics of patients and controls are listed in . All patients were in stable clinical status at the time of this study, without signs of acute infection or acute cardiac problems. History of cardiovascular disease (CVD) was taken from medical records of each patient.
Table 1. Clinical and laboratory data of control subjects and patients with decreased renal function
Patients with chronic kidney diseases
The group of patients with chronic kidney diseases not yet dialyzed (CHRI group) consisted of 45 patients. Their glomerular filtration rate (GFR) ranged from 6 to 128 mL/min/1.73 m2 (median 23 mL/min/1.73 m2), and proteinuria ranged from 0.04 to 11.6 g/24 h. The duration of their renal diseases was from a minimum of 5 months to a maximum of 31 years. Causes of nephro- pathy were immunoglobulin A nephritis in 10 patients, membranous nephropathy in 2 patients, hypertensive nephropathy in 13 patients, diabetic nephropathy in 3 patients, interstitial nephritis in 7 patients, cystic kidney disease in 6 patients, and multifactorial in 4 patients. The majority of the patients had hypertension and were treated with moderate doses of antihypertensive drugs. Twelve patients had CVD and 14 patients had type 2 diabetes mellitus (DM) treated with insulin or peroral antidiabetics. Twenty-six patients had dyslipidemia and were treated with statins.
Patients with end-stage renal disease treated with HD
The HD group consisted of 31 long-term HD patients. Causes of renal failure were as follows: glomerulonephritis in 4 patients, hypertensive nephropathy in 4 patients, cystic kidney disease in 7 patients, interstitial nephritis in 5 patients, diabetic nephropathy in 4 patients, and multifactorial in 7 patients. Their mean residual diuresis was 660 ± 694 mL/24 h. The majority of patients were dialyzed three times/week for 4 h and their dialysis treatment lasted for 3 months to 17 years. They received 1500 ± 658 IU heparin per session, their mean ultrafiltration rate was 598 mL/h, and Kt/V 1.46 ± 0.2. HD treatment was performed using conventional bicarbonate-buffered dialysate in all patients. Of all patients, 91% used native arteriovenous fistulae for dialysis; in other cases arteriovenous fistulae with artificial graft were used. Ten patients were dialyzed with high-flux dialyzers and in the rest of the group low-flux dialyzers were used. Dialyzers were made of poly-sulfone (35.5%), diacetate cellulose (48.3%), and triacetate cellulose (16.2%). The majority of the patients had hypertension and were treated with moderate doses of antihypertensive drugs. Eight patients had type 2 diabetes treated with insulin or peroral antidiabetics. Thirteen patients had CVD and 8 patients had DM type 2 treated with insulin or peroral antidiabetics. Fourteen patients had dyslipidemia treated by statins. They were administered an average weekly erythropoietin 109 IU/kg body weight.
Control group
The control group consisted of 38 matched for age-healthy subjects. They were not administered any special alimentary supplements at the time of this study.
This study was performed in adherence to the principles of the Declaration of Helsinki and approved by the Institutional Ethical Committee. All participants gave their informed consent prior to entering this study.
Samples
In HD patients, blood was collected by puncturing the arteriovenous fistula before starting the dialysis session and prior to heparin administration. In other subjects, blood was collected after overnight fasting by puncturing the cubital vein, with simultaneous blood collection for routine control examinations. Blood was centrifuged for 10 min at 1450 g, and serum was frozen at –80°C. Additionally, in about half of the patients with renal insufficiency not yet dialyzed, a 24-h urine sample was collected, frozen, and used for analysis. Analysis of all samples was performed within 6 months after collection.
Laboratory Parameters
PlGF assay
PlGF was measured by means of sandwich enzyme-linked immunosorbent assay using standard kits (R&D Systems, Inc., Minneapolis, MN, USA, www.RnDSystems.com) according to the manufacturer's protocol. Results are given in picogram per milliliter (pg/mL).
Other parameters
CRP was determined using turbidimetry. GFR was calculated by 24-h urine collection. Routine biochemical parameters were determined by standard clinical chemistry methods using automated analyzers.
Statistical Analysis
Results are expressed as mean ± SD. Analysis of variance (ANOVA), Mann–Whitney test, and t-test were used for evaluation of differences among groups. Associations between parameters were determined by using Pearson or Spearman correlation coefficients. All results are considered statistically significant at p < 0.05.
RESULTS
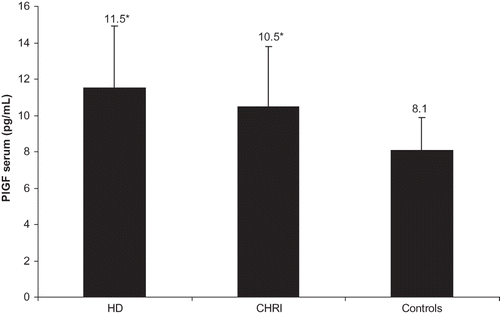
PlGF levels were significantly elevated in both CHRI and HD groups compared to healthy subjects, without significant differences between CHRI and HD patients (10.5 ± 3.3 pg/mL in CHRI patients and 11.5 ± 3.4 pg/mL in HD patients vs. 8.1 ± 1.8 pg/mL in controls, both p < 0.0001 vs. controls, ). Even in a subgroup of 16 patients with mild and marked renal insufficiency, mean GFR 54 ± 24 mL/min/1.73 m2, PlGF was elevated when compared with control subjects (9.9 ± 2.8 pg/mL vs. 8.1 ± 1.8 pg/mL, p < 0.002). Concerning HD patients, no difference of serum PlGF levels between patients using low-flux (n = 21) and high-flux (n = 10) membranes was shown (11.3 ±2.9 vs. 12.1 ± 2.9, p = 0.47).
Figure 1. Serum PlGF levels in patients with chronic kidney disease and various degrees of decreased renal function (CHRI, HD patients and healthy subjects). Notes: Results expressed as mean ± SD. *p < 0.0001, HD and CHRI versus controls.
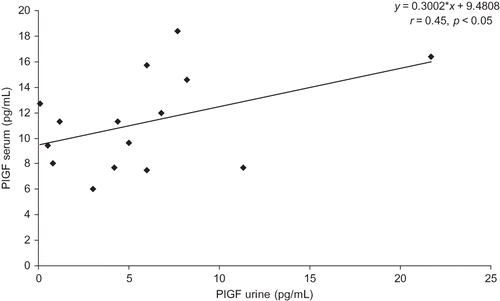
In 15 of the 19 patients with nephropathy, PlGF was detectable in urine samples (mean 5.8 ± 5.4 pg/mL) and correlated with its serum levels, r = 0.45, p < 0.05, .
No relationship between PlGF and serum creatinine concentrations was found in CHRI patients. In CHRI patients, the negative correlation of PlGF with diuresis (r = −0.28) was of borderline significance (p = 0.06). In HD patients, the negative correlation between serum PlGF levels and residual diuresis was not significant (r = −0.43, p = 0.18). There was no correlation of serum PlGF to proteinuria in CHRI patients.
Higher levels of PlGF were found in CHRI patients with CVD, compared with those free of such complication (12.1 ± 3 pg/mL vs. 10.0 ± 3.2 pg/mL, p = 0.03). The differences of PlGF in HD patients with CVD and those HD patients without this complication were not significant (11.8 ± 2.3 pg/mL vs. 11.2 ± 4.0 pg/mL, p = 0.48).
No difference in serum PlGF levels was observed between patients with DM and those without DM in both CHRI patients (10.1 ± 3.5 pg/mL vs. 11.4 ± 2.4 pg/mL, p = 0.15) and HD patients (12.5 ± 2.5 pg/mL vs. 11.1 ± 3.6 pg/mL, p = 0.23).
We found that PlGF in CHRI group correlated significantly with triglyceride concentrations (r = 0.32, p < 0.05) and in HD group correlated significantly with LDL concentrations (r = 0.36, p < 0.05). In addition, serum levels of PlGF were not significantly different among patients with dyslipidemia treated with statins and those without this treatment in both CHRI and HD groups. PlGF also correlated with age in CHRI group (r = 0.35, p = 0.02); correlations in controls (r = 0.30) was of borderline significance (p = 0.06). PlGF was not associated with CRP levels in any of the studied groups.
In summary, PlGF levels are increased in patients with renal impairment with decreased renal function including hemodialysis patients. The increase could have been demonstrated even in the subgroup of patients with mild and marked renal insufficiency. PlGF is also present in the urine, and serum and urine concentrations of PlGF are significantly interrelated. It is higher in CHRI patients with cardiovascular disease. In HD patients, PlGF levels are related to LDL cholesterol concentrations as a risk marker of atherosclerosis, but not to CRP as an inflammatory marker.
DISCUSSION
In this study, in 45 patients with chronic kidney disease with different degrees of renal insufficiency not yet dialyzed and 31 hemodialysis patients, we noted elevated PlGF levels compared with healthy controls. The serum levels of PlGF in HD patients do not differ from the PlGF concentrations in patients with various degrees of decreased renal function not yet dialyzed. Additionally, this is the first study where PlGF levels were measured simultaneously in serum and urine in patients with decreased renal function. PlGF is present in the urine of patients with renal impairment, and serum and urine concentrations of PlGF are significantly interrelated in patients with decreased renal function. Given that PlGF is a small dimeric protein with a molecule (≈50 kDa),Citation7 it is readily filtered into urine even in the absence of renal damage.Citation24 PlGF is derived entirely from the circulating blood and not from the cells of the kidney itself (glomerular podocytes and tubular cells) as urinary VEGF.Citation25,Citation26 Although no relationship between PlGF and serum creatinine concentrations was found in CHRI patients, serum PlGF levels tended to correlate with diuresis in CHRI and HD patients.
PlGF expression increases significantly in early gestation, peaks at around 26–30 weeks, and decreases as term approaches.Citation27 PlGF has also been used as a predictor of the common pregnancy-associated hypertensive disorder preeclampsia. Plasma, serum, and urine PlGF levels decrease significantly in women with preeclampsia and/or those who subsequently develop the disorder.Citation27–30 Serum PlGF levels in nonpregnant subjects (both males and females) are much lower in comparison with pregnant women. PlGF mRNA is present in very small amounts in heart, lung, thyroid, goiter, and skeletal muscle. It is not expressed, however, in kidney and pancreas.Citation31,Citation32
PlGF was recently shown to be upregulated in early and advanced atherosclerotic lesions.Citation23,Citation33 Irrespective of its potential role in the development of atherosclerosis, in this study, increased plasma PlGF levels obviously characterize patients with renal disease as a separate population when compared with normal subjects without renal and cardiac disease. In contrast, in a recent study examining the role played by soluble Fms-like kinase-1 (sFlt-1), an endogenous antagonist of the proatherogenic cytokine PlGF, authors demonstrated that plasma PlGF levels were unaffected by differences in renal function. Although the PlGF/sFlt-1 ratio was negatively correlated with the estimated glomerular filtration rate, plasma PlGF levels were not affected by it.Citation34
In addition, our study showed higher levels of PlGF in CHRI patients not yet dialyzed with CVD compared to those patients without CVD. This finding suggests that PlGF might be an indicator of CVD and atherosclerotic complications in patients with decreased renal function. This finding is in line with the finding of Onoue et al.,Citation34 where the PlGF/sFlt-1 ratio was significantly higher in patients with multivessel coronary artery disease than in patients with single-vessel or no coronary disease. In the animal part of this study, a reduction in the circulating levels of sFlt-1 was associated with the worsening of atherosclerosis that accompanied renal dysfunction, whereas serum PlGF concentrations were higher in the 5/6-nephrectomized mice. In the peripheral circulation, free PlGF, free sFLT-1, and the PlGF-sFlt-1 complex are present simultaneously. The PlGF–sFlt-1 complex was most closely correlated with the severity of atherosclerosis, in both patients with renal dysfunction and 5/6-nephrectomized mice.Citation34
In a cohort of 190 type 1 diabetic patients with diabetic nephropathy, elevated PlGF levels predicted higher risk of coronary heart disease after 10 years of follow-up, independent of kidney function and established coronary heart disease risk biomarkers.Citation35 In this study, the PlGF levels in patients with decreased renal function with DM and those patients without DM were not different. The reason might be due to the relatively small sample size of patients with DM in this study.
In the general population, elevated PlGF levels showed a modest correlation between PlGF levels and triglyceride concentrations, and inverse correlation with high-density lipoprotein levels among women without known coronary heart disease.Citation36 This study suggested that elevated PlGF levels might be associated with subsequent risk of coronary heart disease. In our study we found a modest relation between plasma PlGF levels and LDLs in hemodialysis patients and triglyceride concentrations in patients with chronic kidney disease. The finding that PlGF is linked to classic risk factors of atherogenesis in our cohort of patients with decreased renal function is a novel finding suggesting that this growth factor might play a role in atherosclerosis in these patients. Furthermore, PlGF might be considered as one of the candidates to be a biomarker for accelerated atherosclerosis, particularly plaque instability, myocardial ischemia, and prognosis of the patients with cardiovascular and renal disease.Citation37
In this study, PlGF levels in HD patients with low-flux and high-flux dialysis membranes were not different, as with larger solutes of 50 kDa the clearance by low-flux, and even high-flux, dialysis is practically nil.Citation38 Therefore, we can also hypothesize that PlGF is not filtered during dialysis due to the size of the protein.Citation7 We can speculate that one source of increased PlGF levels in HD patients might be monocytes activation during long-term hemodialysis.
Circulating levels of VEGF and PLGF are increased in animal models of sepsis.Citation39 Recently the same authors suggested that upregulation of PlGF in sepsis is an adaptive host response that exerts its benefit, at least in part, by attenuating VEGF signaling,Citation40 implicating that the effects of PlGF are highly context dependent. In our study, there was no correlation between serum PlGF levels and CRP. Although CRP levels were slightly higher in the studied groups, PlGF levels were low and patients were in a stable clinical state. These factors might be the reason that no relationship of PlGF and CRP, a marker of micro-inflammation, was found.
Statins exert cardioprotective actions partly through anti-inflammatory actions, in addition to their lipid-lowering effects.Citation41,Citation42 Recently it was shown that atorvastatin increased plasma levels of sFlt-1, whereas decreasing VEGF and PlGF levels. These changes were associated with late improvement of post-myocardial infarction ventricular function and might represent an additional benefit of statin therapy.Citation43 Although there were no differences in PlGF levels of CHRI and HD patients with and without statin therapy in this study probably due to small sample size, the beneficial effect of lipid-lowering therapy on PlGF levels cannot be excluded in patients with chronic kidney disease.
Atherosclerosis is more commonly observed in the elderly than in the young. In the general population it was shown that PlGF weakly correlated with age.Citation36 In this study there was a modest correlation between serum PlGF levels and age in the CHRI group and the control group. The correlation in the control group was of borderline significance; in the HD group the correlation with age was not significant probably due to the small sample size and other possible influencing factors. Renal insufficiency and worsening of atherosclerosis is a complex process related to renal dysfunction and ageing, and many factors are still unknown.
In conclusion, PlGF, an endogenous proatherogenic cytokine, is elevated in patients with decreased renal function. This study also shows that PlGF is present in the urine, and its serum and urine levels are interrelated. It is higher in CHRI patients with cardiovascular disease. This finding warrants further investigation to understand PlGF's importance in atheroma formation and plaque destabilization and to determine its utility as a long-term risk biomarker in patients with kidney disease.
ACKNOWLEDGMENTS
The authors are thankful to laboratory staff Mrs. Dita Hudcová, Mrs. Helena Miškovská, and MSc. Jana Švarcová for technical assistance. The authors are equally thankful to nurses from outpatient department and dialysis center, especially to Bc. Martina Růžičková, Mrs. Hana Čejková, and Bc. Magdalena Bartková.
Declaration of interest: This study was supported by grant from the Internal Grant Agency of the Czech Ministry of Health NS/10043-4/2008.
REFERENCES
- Ma KW, Green EL, Raij L. Cardiovascular risk factors in chronic renal failure and hemodialysis populations. Am J Kidney Dis. 1992;19:505–513.
- London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678–1695.
- Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl. 3):112–119.
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126.
- Foley RN, Parfrey PS, Harnett JD, Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192.
- Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88:9267–9271.
- Iyer S, Leonidas DD, Swaminathan GJ, The crystal structure of human placental growth factor-1 (PlGF-1) an angiogenic protein, at 2.0 A resolution. J Biol Chem. 2001;276:12153–12161.
- Yang W, Ahn H, Hinrichs M, Torry RJ, Torry DS. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J Reprod Immunol. 2003;60:53–60.
- Maglione D, Guerriero V, Viglietto G, Two alternative mRNAs coding for the angiogenic factor, placenta growth factor (PlGF), are transcribed from a single gene of chromosome 14. Oncogene. 1993;8:925–931.
- Cao Y, Ji WR, Qi P, Rosin A, Cao Y. Placenta growth factor: Identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem Biophys Res Commun. 1997;235:493–498.
- Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: Implications for abnormal placentation. J Soc Gynecol Investig. 2003;10:178–188.
- Vuorela P, Hatva E, Lymboussaki A, Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997;56:489–494.
- Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: Novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Hemost. 2003;1:1356–1370.
- Chen CN, Hsieh FJ, Cheng YM, The significance of placental growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73–82.
- Ho MC, Chen CN, Lee H, Placenta growth factor not vascular endothelial growth factor A or C can predict the early recurrence after radical resection of hepatocellular carcinoma. Cancer Lett. 2007;250:237–249.
- Parr C, Watkins G, Boulton M, Cai J, Jiang WG. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41:2819–2827.
- Carmeliet P, Moons L, Luttun A, Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravagation in pathological conditions. Nat Med. 2001;7:575–583.
- Failla CM, Odorisio T, Cianfarani F, Schietroma C, Puddu P, Zambruno G. Placenta growth factor is induced in human keratinocytes during wound healing. J Invest Dermatol. 2000;115:388–395.
- Oura H, Bertoncini J, Velasco P, Brown LF, Carmeliet P, Detmar M. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood. 2003;101:560–567.
- Maes C, Coenegrachts L, Stockmans I, Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest. 2006;116:1230–1242.
- Perelman N, Selvaraj SK, Batra S, Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102:1506–1514.
- Luttun A, Tjwa M, Moons L, Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840.
- Levine RJ, Thadhani R, Qian C, Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293(1):77–85.
- Brown LF, Berse B, Tognazzi K, Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 1992;42:1457–1461.
- Simon M, Gröne HJ, Jöhren O, Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250.
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544.
- Levine RJ, Maynard SE, Qian C, Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683.
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: Evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182.
- Livingstone JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol. 2000;183:1554–1557.
- Maglione D, Guerriero V, Viglietto G, Two alternative mRNAs coding for the angiogenic factor, placenta growth-factor (PlGF), are transcribed from a single gene of chromosome 14. Oncogene. 1993;8:925–931.
- Ziche M, Maglione D, Ribatti D, Placenta growth factor-1 is chemotactic, mitogenic and angiogenic. Lab Invest. 1997;76:517–531.
- Khurana R, Moons L, Shafi S, Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation. 2005;111:2828–2836.
- Onoue K, Uemura S, Takeda Y, Reduction of circulating soluble fms-like tyrosine kinase-1 plays a significant role in renal dysfunction-associated aggravation of atherosclerosis. Circulation. 2009;120:2470–2477.
- Tarnow L, Astrup AS, Parving HH. Elevated placental growth factor (PlGF) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Scand J Clin Lab invest Suppl. 2005;240:74–79.
- Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ, Rimm EB. Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol. 2009;29:134–139.
- Apple FS, Wu AH, Mair J, Committee on Standardization of Markers of Cardiac Damage of the IFCC. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51:810–824.
- Cheung AK. Quantitation of dialysis. The importance of membrane and middle molecules. Blood Purif. 1994;12:42–53.
- Yano K, Liaw PC, Mullington JM, Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203:1447–1458.
- Yano K, Okada Y, Beldi G, Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med. 2008;205:2623–2631.
- Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering – are they clinically relevant? Eur Heart J. 2003;24:225–248.
- Ray KK, Cannon CP. Intensive statin therapy in acute coronary syndromes: Clinical benefits and vascular biology. Curr Opin Lipidol. 2004;15:637–643.
- Kodama Y, Kitta Y, Nakamura T, Atorvastatin increases plasma soluble Fms-like tyrosine kinase-1 and decreases vascular endothelial growth factor and placental growth factor in association with improvement of ventricular function in acute myocardial infarction. J Am Coll Cardiol. 2006;48:43–50.