Abstract
Background: Both erythropoiesis-stimulating agents and iron treatments are underutilized in peritoneal dialysis (PD) patients. Studies to evaluate safety profiles of various intravenous iron preparations are limited in PD patients compared to hemodialysis. No study in the literature compared safety of low-molecular-weight iron dextran (LMW-ID) with that of iron sucrose in PD patients. We aimed to compare adverse-effect profiles of LMW-ID and iron sucrose with varying dosing schedules in PD patients with a hope to foster use of parenteral iron solutions in PD patients. Methods: We retrospectively reviewed patient charts and included patients who were administered iron sucrose or LMW-ID parenterally. Sociodemographic characteristics, clinical features, and pertinent laboratory data were collected. Adverse events which were deemed to be related to infusion of parenteral iron were recorded. We double-checked both physician records and nursing documents for observed adverse events. Results: A total of 167 chronic PD patients were included in the study, and 92 patients were administered LMW-ID, whereas 75 patients were administered iron sucrose. Only one adverse event occurred in a patient who was administered 500 mg iron sucrose in a single infusion. Conclusions: This study showed the comparable safety of LMW-ID in varying doses over that of iron sucrose in PD patients.
INTRODUCTION
Anemia is an almost universal complication of chronic kidney disease, and majority of patients undergoing renal replacement therapies receive anemia treatment.Citation1 Treatment of anemia in end-stage renal disease patients is mainly composed of two components: erythropoiesis-stimulating agents (ESAs) and iron. For a number of reasons, peritoneal dialysis (PD) patients are less severely anemic compared to hemodialysis counterparts.Citation2 Nevertheless, given current recommendations of guideline targets, still a significant proportion of PD patients require anemia treatment at some time during their follow-up. Both ESA and iron treatments are underutilized in PD patients.Citation3 Iron requirements are increased especially when ESAs are used due to increased demand of bone marrow to produce new red cells. Efficacy of parenteral iron against oral supplementation has been demonstrated in both hemodialysis and PD patients.Citation4 Compared to hemodialysis, the issue of safety and efficacy of parenteral iron therapy in PD patients has been relatively ignored to date. Most of the studies are outdated and suffer from small sample sizes. In addition, newer and safer intravenous iron preparations have not been studied in PD patients. To our knowledge, no study in the literature compared the safety of low-molecular-weight iron dextran (LMW-ID) with that of iron sucrose in PD patients. Therefore, we aimed to compare the adverse-effect profiles of LMW-ID and iron sucrose with varying dosing schedules in PD patients with a hope to foster use of parenteral iron solutions in PD patients.
MATERIALS AND METHODS
This retrospective study was conducted at the outpatient PD clinic and inpatient nephrology clinic at Meram School of Medicine Hospital in Konya, Turkey. We retrospectively reviewed patient charts and included patients who were administered iron sucrose or LMW-ID parenterally. Sociodemographic characteristics, clinical features, and pertinent laboratory data were collected. Adverse events which were deemed to be related to infusion of parenteral iron were recorded. We double-checked both physician records and nursing documents for observed adverse events. Laboratory data including serum iron, iron-binding capacity, ferritin, hemoglobin, C-reactive protein, urea, creatinine, and parathyroid hormone were obtained from the charts.
Iron sucrose or LMW-ID was chosen on preferences of caring nephrologists and availability of the specific preparation at the hospital chemist at that time. Dose of the specific formulation was calculated as the need for elemental iron. Specific form of the iron solution and infusion number and amount of elemental iron administered at each infusion were based on the preference of the attending physician. Commercial preparations of Cosmofer (Say İlaç Sanayi ve Ticaret Ltd.Şti, Istanbul, Turkey) and Venofer (Abdi İbrahim İlaç San ve Tic A.Ş., Istanbul, Turkey) were used as LMW-ID and iron sucrose, respectively. Each 2 mL LMW-ID ampule consists of 625 mg Fe(III)-hydroxy dextran which is equivalent of 100 mg elemental iron, whereas each 5 mL ampule of iron sucrose consists of 2700 mg Fe(III)-hydroxide in sucrose which is equivalent to 100 mg elemental iron.
Parenteral iron infusion protocol at our clinics was as follows: first, parenteral solution containing 25 mg elemental iron was slowly infused under close supervision as test dose. If no allergic reaction was observed 20 min after completion of test dose, the iron solution was infused. Both parenteral iron solutions were diluted in normal saline. Iron solutions were diluted in 250 mL normal saline when 100–500 mg elemental iron was administered in a single infusion. When 500–1000 mg elemental iron was administered in a single infusion, 500 mL normal saline was used. Total infusion times were 3 h and 6 h for 250 mL and 500 mL infusion solutions, respectively. Unlike near-universal use of 125 mg prednisolone prior to and after infusion of iron solution in the United States, we did not administer prednisolone, paracetamol, or antihistaminics to the patients on a regular basis. There were no repeated doses of iron sucrose or iron dextran in PD patients.
RESULTS
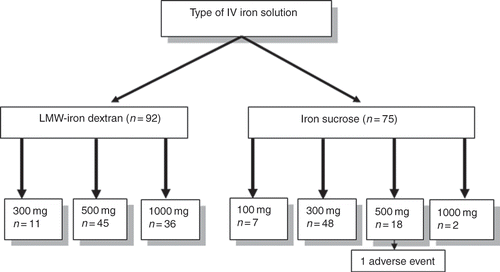
A total of 167 chronic PD patients were included in the study. While 92 patients were administered LMW-ID, 75 patients were administered iron sucrose. Mean age, gender distribution, and laboratory data of LMW-ID and iron sucrose groups are shown in . Different dosing schedules and numbers of each parenteral iron formulation are seen in . We observed only one adverse event which occurred in a patient who was administered 500 mg iron sucrose in a single infusion. The patient developed generalized pruritus after 10 min of infusion. After stopping infusion and application of 125 mg methyl prednisolone intravenously, the adverse reaction quickly lessened and disappeared. There was no other adverse event in the patient population.
Table 1. Laboratory data of chronic peritoneal dialysis patients administered LMW-ID and iron sucrose
DISCUSSION
The main result of this study is that intravenous LMW-ID and iron sucrose are safe and comparable in terms of adverse reactions in PD patients. Only one adverse reaction was observed in iron sucrose group, which was mild and transient in nature.
PD patients usually have milder degrees of anemia compared to hemodialysis patients. Nevertheless, 80–90% of patients still require anemia treatment based on guideline recommendations.Citation5 Intravenous iron was shown to facilitate anemia management and reduce ESA doses and the cost of the treatment.Citation6 Despite widespread use in hemodialysis patients, intravenous iron is apparently underutilized in PD patients. In their large survey, St. Peter et al.Citation7 investigated patterns of intravenous iron use from 1997 to 2002 in the United States. They showed that despite an increase in iron usage in PD patients from 5.6% in 1994 to 19.3% in 2002, these percentages clearly lagged behind hemodialysis patients (42.2% in 1994 and 84.4% in 2002). A number of factors can be held responsible for this practice. First, anemia is somewhat milder in PD patients. This is the result of a combination of presence of a fair amount of residual renal function, lower amount of iron loss from the body, and more stable hemodynamic status (in hemodialysis patients, between the dialysis sessions, serum hemoglobin values can swing as much as 2–3 g/dL). Second, historically oral iron has been used more commonly in PD patients than in hemodialysis counterparts. This may have hampered intravenous iron use coupled with milder degree, more easily manageable anemia in PD patients. However, as in the hemodialysis patients, superiority of parenteral iron in PD patients was documented.Citation4 Third, adverse reactions, especially anaphylaxis and fatalities that might be seen with iron dextran, may have a negative role on physician behavior. Fourth, PD patients are usually followed up by 1- or 2- month cycles at outpatient clinics. This relatively rare attendance to hospital or clinic and lack of a readily available vascular access may also be responsible for underutilization of intravenous iron in PD patients. Lastly, some theoretical concerns about the potential role of parenteral iron in oxidative stress and infection risk (especially peritonitis), despite refutal by some studies,Citation8 may have restrained parenteral iron use in PD patients.
Until 1999, the only available iron preparation in the market in the United States was iron dextran (high molecular weight). Albeit not so commonly seen, reports of anaphylaxis and death led to reluctance by many caregivers to use intravenous iron. This safety risk transformed into search for alternative routes of administration of iron in PD patients. Oral route gained popularity despite high rates of gastrointestinal intolerance.Citation9 Alternatively, the thought of administering iron through peritoneal cavity emerged. Experimental studies revealed that parenteral iron was not toxic to the peritoneal membrane.Citation10–12 In a pilot study Mars and coworkersCitation13 concluded that the use of bolus intraperitoneal iron dextran was safe, effective, and convenient and did not demonstrate any harmful effect on the integrity of the peritoneal membrane. However, attention of the medical community turned away from this practice quickly and the procedure did not enjoy a widespread acceptance.
In 1999, two new preparations, ferric gluconate and iron sucrose, were approved for use in the United States. Anaphylactoid reactions were rarely seen with these products compared with iron dextran. Thus, ferric gluconate and iron sucrose constituted the majority of intravenous iron prescribed in hemodialysis patients.Citation7 Despite the presence of safer alternatives to iron dextran, there are only a handful of studies in the literature that investigate relative safety of these newer preparations in PD patients. One of these studiesCitation14 compared the safety and efficacy of high-molecular-weight iron dextran with that of iron sucrose. Both agents were equally effective in restoring iron stores. However, of 23 patients who were administered iron sucrose, 1 developed anaphylaxis and 2 had transient chest pain. Iron dextran group had more adverse reactions overall (7.4% of injections and one anaphylaxis at test dose vs. 4.3% in iron sucrose group). Another small study comprising 17 chronic PD patients found adverse reaction rates of 0.9% and 5.9% for 100 mg and 200 mg infusions of iron sucrose, respectively.Citation15 In their randomized controlled trial, Li and WangCitation16 randomized 46 PD patients into intravenous iron sucrose (26 patients, 200 mg iron sucrose per week) and oral ferrous succinate 300 mg three times a day. After 8 weeks of treatment, there were no adverse events with intravenous iron. The authors found intravenous iron was more effective to correct anemia compared with oral iron supplementation. A recent multicenter trial in which 188 PD patients on ESA therapy were administered 1 g iron sucrose in three divided doses or no iron at all, there was only one adverse event (pruritus and swelling of the feet) in iron sucrose treated patients.Citation17
We did not find any allergic reaction related to LMW-ID whereas only one iron sucrose treated patient developed a mild and transient adverse event. This event rate for iron sucrose use is clearly less than those previously reported in PD patients. However, this rate is generally in agreement with that reported for hemodialysis patients.Citation18
To the best of our knowledge, there is no study in the literature evaluating the safety profile of LMW-ID in PD patients to date. LMW-ID was devised after recognizing that the culprit for allergic reactions was carbohydrate coating (or shell) used in parenteral iron preparations.Citation19 High-molecular-weight dextran can cause anaphylaxis due to large chained molecular structure. In LMW-ID, molecular weight has been dramatically reduced along with allergic adverse events. Safety and efficacy of LMW-ID, despite still being a dextran, has been demonstrated in a number of studies and surveys in hemodialysis and chronic kidney disease patients.Citation20–22 The results of this study extend this knowledge to cover PD patients also. One advantage of LMW-ID over iron sucrose is the availability of total dose infusion in which all the calculated amount of iron is given in a single infusion.Citation23 Of course, this is particularly important in PD population who do not have a permanent vascular access and attend outpatient dialysis clinics less frequently than hemodialysis patients. Thus, an iron solution with a less allergic profile and the ability to be administered in a single infusion would be a beneficial stimulus for both patients and the health-care team to increase the rate of parenteral iron use.
This study has a few limitations that deserve to be mentioned. Retrospective design is subjected to possible errors while gathering info from patient charts. However, we double-checked adverse events from two independent sources: patient charts and nursing follow-up documents. Thus, the likelihood to overlook an adverse event related to iron infusion seems very low. Unlike the total number, the number of patients in each different dose schedule may be relatively small. This may be a hurdle to confidently say all dosing schedules studied here are entirely safe. Despite these weaknesses, this study is the first to evaluate and compare the safety of LMW-ID and iron sucrose in chronic PD patients.
In conclusion, this study showed the comparable safety of LMW-ID in varying doses over that of iron sucrose in PD patients. As with hemodialysis, studies conducted in a prospective manner are a clear need to evaluate the safety of newer-generation iron preparations in PD patients. Confirming the safety may be a stimulus, among other things, to increase the use of parenteral iron in PD patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Locatelli F, Del Vecchio L, Pozzoni P. Treating anemia at different stages of renal disease. J Nephrol. 2007;20(Suppl. 12): S33–S38.
- Durand PY. Iron therapy: Is it different with peritoneal dialysis compared to hemodialysis? Nephrol Ther. 2006;2(Suppl. 5): S341–S346.
- U.S. Renal Data System (USRDS). 2006 annual data report: Atlas of end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA; 2006.
- Ahsan N. Infusion of total dose iron versus oral iron supplementation in ambulatory peritoneal dialysis patients: A prospective, cross-over trial. Adv Perit Dial. 2000;16:80–84.
- Macdougall IC. How to optimise anaemia therapy in peritoneal dialysis patients. Contrib Nephrol. 2006;150:202–213.
- Kapoian T, O'Mara NB, Singh AK, Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19(2):372–379.
- St. Peter WL, Obrador GT, Roberts TL, Collins AJ. Trends in intravenous iron use among dialysis patients in the United States (1994–2002). Am J Kidney Dis. 2005;46(4):650–660.
- Allen JR, Troidle LK, Juergensen PH, Kliger AS, Finkelstein FO. Incidence of peritonitis in chronic peritoneal dialysis patients infused with intravenous iron dextran. Perit Dial Int. 2000;20(6):674–678.
- Wingard RL, Parker RA, Ismail N, Hakim RM. Efficacy of oral iron therapy in patients receiving recombinant human erythropoietin. Am J Kidney Dis. 1995;25(3):433–439.
- Bastani B, Galley S. Intraperitoneal iron-dextran as a potential route of iron therapy in CAPD patients. Perit Dial Int. 1996;16(6):646–648.
- Pecoits-Filho RF, Twardowski ZJ, Kim YL, Khanna R, Morre H, Nolph KD. The absence of toxicity in intraperitoneal iron dextran administration: A functional and histological analysis. Perit Dial Int. 1998;18(1):64–70.
- Reddy DK, Moore HL, Lee JH, Chronic peritoneal dialysis in iron-deficient rats with solutions containing iron dextran. Kidney Int. 2001;59(2):764–773.
- Mars RL, Moles K, Pope K, Hargrove P. Use of bolus intraperitoneal iron dextran in continuous ambulatory peritoneal dialysis or continuous cyclic peritoneal dialysis patients receiving recombinant human erythropoietin. Adv Perit Dial. 1999; 15:60–64.
- Prakash S, Walele A, Dimkovic N, Bargman J, Vas S, Oreopoulos D. Experience with a large dose (500 mg) of intravenous iron dextran and iron saccharate in peritoneal dialysis patients. Perit Dial Int. 2001;21(3):290–295.
- Vychytil A, Haag-Weber M. Iron status and iron supplementation in peritoneal dialysis patients. Kidney Int Suppl. 1999; 69:S71–S78.
- Li H, Wang SX. Intravenous iron sucrose in peritoneal dialysis patients with renal anemia. Perit Dial Int. 2008;28(2):149–154.
- Singh H, Reed J, Noble S, Cangiano JL, Van Wyck DB. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: A randomized, controlled trial. Clin J Am Soc Nephrol. 2006; 1(3):475–482.
- Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21(2):378–382.
- Macdougall IC. Evolution of iv iron compounds over the last century. J Ren Care. 2009;35(Suppl. 2):8–13.
- Sav T, Tokgoz B, Sipahioglu MH, Is there a difference between the allergic potencies of the iron sucrose and low molecular weight iron dextran? Ren Fail. 2007;29(4):423–426.
- Sinha S, Chiu DY, Peebles G, Comparison of intravenous iron sucrose versus low-molecular-weight iron dextran in chronic kidney disease. J Ren Care. 2009;35(2):67–73.
- Auerbach M, Al Talib K. Low-molecular weight iron dextran and iron sucrose have similar comparative safety profiles in chronic kidney disease. Kidney Int. 2008; 73(5):528–530.
- Auerbach M, Rodgers GM. Intravenous iron. N Engl J Med. 20075;357(1):93–94.