Abstract
Background: Patients undergoing maintenance hemodialysis (MHD) have a high prevalence of peptic ulcer disease (PUD). Omeprazole is a proton pump inhibitor with proven efficacy in the prevention and treatment of PUD. However, there is little data on the prophylactic use of omeprazole in reducing the risk of PUD among MHD patients. Methods: This prospective study included 93 patients undergoing MHD at Zen-Ho Dialysis Center between July 2008 and December 2009. Fifty-three patients were assigned to receive 20 mg of omeprazole daily for 18 months and 40 patients served as control. The Kaplan–Meier method was applied to calculate the cumulative incidence of PUD. Results: The per-protocol population comprised 85 patients (omeprazole group, 49; control group, 36). Both groups had similar baseline characteristics. The need for endoscopy was found to be significantly less (10.2 vs. 44.4%, p = 0.001) in the omeprazole group than in the control group. Dialysis patients in the omeprazole group required fewer blood transfusions and erythropoietin doses than did the control group patients. Kaplan–Meier analysis revealed a higher cumulative ulcer rate in the control group (log-rank test, p = 0.04). However, omeprazole did not reduce the risk of PUD in MHD patients on regular aspirin or warfarin. Conclusions: We conclude that prophylactic use of omeprazole might be effective to lower the incidence of PUD among MHD patients without regular aspirin or warfarin use. Further large-scale controlled trials should be carried out to confirm our findings.
INTRODUCTION
Dialysis patients are known to have an elevated bleeding risk. Although the pathogenesis of excessive bleeding in patients with end-stage renal disease is multifactorial, defects of platelet function and platelet–vessel wall interaction are thought to play a major role.Citation1,Citation2 Moreover, because of a high risk of cardiovascular or cerebrovascular diseases in dialysis patients, a high prevalence of antiplatelet or warfarin use further contributes to an increased bleeding risk.Citation2 It has been reported that a majority of the bleeding events originate from the gastrointestinal (GI) tract.Citation2,Citation3 Gastroduodenal ulcers, that is, peptic ulcer disease (PUD), have been reported to account for nearly 60% of upper GI bleeding episodes among patients receiving maintenance hemodialysis (MHD).Citation3,Citation4 Systemic/local circulatory failure, hypergastrinemia, high ammonia levels, and enhanced inflammation were considered to contribute to GI mucosal injury in MHD patients.Citation1,Citation4 Even though more and more studies explore this topic, several important issues still remain unsettled.
Several reports have indicated that dialysis patients, despite having a lower prevalence of Helicobacter pylori infection, have a higher occurrence of PUD than those without renal failure.Citation5,Citation6 As dialysis patients have a high risk of GI mucosal damage, these patients with PUD should be managed as a high-risk group,Citation1 and strategies to reduce its incidence need to be developed. Omeprazole is a proton pump inhibitor (PPI) widely used in the treatment of PUD or gastroesophageal reflux disease.Citation7 By blocking the production of gastric acid, omeprazole helps in healing the ulcerated gastric mucosa and relieving dyspepsia.Citation7 The efficacy of PPIs in the prevention of nonsteroidal anti-inflammatory drug-associated ulcers has been largely established in the general population.Citation8–10 Accumulating studies have also shown PPIs to have good safety and efficacy in dialysis patients with PUD.Citation11 However, prophylactic use of omeprazole to reduce the risk of PUD has rarely been reported among MHD patients. Therefore, the primary objective of this study was to determine whether MHD patients on omeprazole have a lower risk for PUD than those not receiving omeprazole. We also assessed the safety and cost-effectiveness of omeprazole use among MHD patients.
MATERIALS AND METHODS
Patient Population and Study Design
This prospective study initially included 124 dialysis patients receiving chronic hemodialysis at Zen-Ho Dialysis Center (Taichung County, Taiwan) between July 2008 and December 2009. Patients were considered eligible for inclusion if they underwent hemodialysis for more than 3 months; they had no GI symptoms at enrollment; and if they agreed to receive an endoscopic examination if either typical GI symptoms for PUD,Citation7 positive fecal occult blood test, or an unexplained hematocrit drop over 3% was noted during the study period. The exclusion criteria were a history of gastric surgery; allergy to omeprazole; use of PPI or histamine2 receptor antagonists within 1 year prior to enrollment; and presence of coagulopathy, thrombocytopenia, liver cirrhosis, or cancer. We also excluded dialysis patients with a history of endoscopically confirmed PUD. Ninety-three eligible subjects were enrolled in this study. Fifty-three patients were assigned to receive 20 mg of omeprazole (Okwe, Nang-Kuang Pharmaceutical Co. Ltd., Tainan County, Taiwan) daily for 18 months, and 40 patients who did not receive omeprazole served as controls. All patients were followed up to investigate the occurrence of PUD during the study period. Patients on medications such as warfarin, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, corticosteroids, and/or antiplatelet drugs prior to the study were allowed to continue use of these medications during the study period. However, new medications were prohibited until the end of the study. The end point was PUD, as defined by a gastric or duodenal ulcer, diagnosed by endoscopy without other identified causes during the 18-month study period. This clinical study followed the principles of the Declaration of Helsinki and was compatible with the policies of the local ethics committee. The anonymity of all enrolled subjects was carefully protected and informed written consent was obtained from all participants.
Collection of Clinical Data
During the study period, a monthly evaluation of complete blood count, serum biochemical data, and fecal occult blood was performed. Hemoglobin and hematocrit levels were obtained bi-weekly and dialysis adequacy (i.e., Kt/V) and parathyroid hormone levels were determined every 3 months. Drug compliance, use of prohibited medications, and drug safety and tolerability were also assessed monthly. Failure to take >80% of the omeprazole doses was considered unsatisfactory compliance. The assessment of safety and tolerability was based on spontaneously reported adverse events and open questionnaires administered by the dialysis staff. We also examined clinical parameters of endoscopically confirmed PUD events, including (1) clinical presentations; (2) endoscopic treatments, findings, and diagnoses; and (3) clinical outcomes (transfusion requirements, complications, and mortality). Blood transfusion was performed in cases if hematocrit levels dropped below 25% or in cases of symptomatic anemia. All the lesions detected by high-definition video endoscopy (EVIS LUCERA GIF-H260; Olympus Optical Co. Ltd., Tokyo, Japan) were examined by experienced endoscopists.Citation12 During endoscopic diagnosis of PUD, all biopsy samples were taken with sterile biopsy forceps to detect H. pylori infection by the rapid urease test. Patients were considered H. pylori-positive if the color change occurred within 24 h. An ulcer was defined as a circumscribed mucosal break at least 5 mm in diameter and having a perceptible depth.Citation7–10 Erosion was defined as a flat mucosal break of any size occurring in the presence of blood in the stomach or duodenum.Citation7–10
Statistical Analysis
Efficacy analyses were carried out only for the per-protocol population. Continuous variables were expressed as means ± standard deviation, and categorical variables were expressed as number or percentage for each parameter, unless otherwise stated. Data were routinely tested for normality of distribution and equality of standard deviations before analysis. All collected hematological and biochemical parameters during the study period were averaged for analysis. For comparison of continuous variables between the omeprazole and control groups, unpaired variables were compared by Student's t-test or Mann–Whitney U-test, and paired variables by Student's t-test or Wilcoxon test, as appropriate. For categorical variables, a cross-table with Fisher's exact test was used. The Kaplan–Meier method was applied to calculate the cumulative incidence of PUD, and the difference was determined by the log-rank test. All statistical analyses were performed using Statistical Packages for Social Sciences (SPSS) 13.0 for Windows (SPSS Inc., Chicago, IL, USA). The criterion for significance was the 95% confidence interval to reject the null hypothesis.
RESULTS
Study Population Characteristics
Ninety-three patients were enrolled in this study and received hemodialysis with a high-flux dialyzer during the study period. In the omeprazole group, one patient discontinued its use because of adverse events and one patient was excluded because of unsatisfactory compliance. During the study period, one patient in the omeprazole group died of malignancy, one in the control group died of sepsis, and one in the control group died of cardiovascular diseases. Additionally, one patient in the omeprazole group and two patients in the control group were excluded from the per-protocol analysis owing to the use of other medications during the study period. Finally, the per-protocol population comprised 85 patients (omeprazole group, 49; control group, 36). As shown in , the omeprazole and control groups were similar with respect to baseline characteristics.
Table 1. Baseline characteristics of the omeprazole and control groups
Gastrointestinal Events
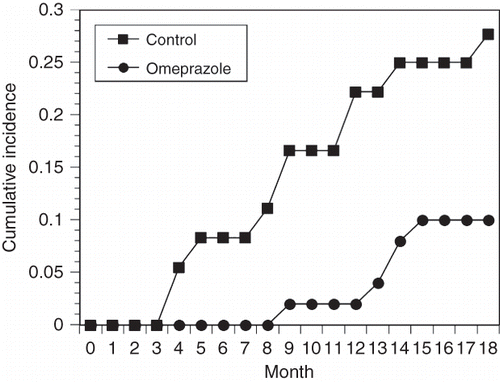
During the study period, 5 patients in the omeprazole group and 16 in the control group underwent endoscopy because of typical symptoms of PUD, a positive fecal occult blood test, or unexplained hematocrit drop over 3% (). Of these, all five patients in the omeprazole group were found to have PUD (two gastric ulcers, two duodenal ulcers, one both gastric and duodenal ulcers). Of the 16 patients in the control group that underwent endoscopy, 15 were found to have PUD (12 gastric ulcers, 6 duodenal ulcers, 3 both gastric and duodenal ulcers). The need for endoscopy was significantly less in the omeprazole group than in the control group (10.2% vs. 44.4%, p = 0.001). Stigmata of recent hemorrhage occurrence were significantly lower in the omeprazole group than in the control group. Among the per-protocol population, the incidence of PUD was higher in the control group (15 patients, 41.7%) than in the omeprazole group (5 patients, 10.2%) (p = 0.001, Fisher's exact test). However, similar H. pylori infection and recurrent bleeding rates were observed between the two groups. Four of the 24 aspirin users in the omeprazole group and 3 of 18 in the control group developed PUD. It is surprising that omeprazole did not reduce the risk of PUD among MHD patients with regular use of aspirin. Similar results were also found in warfarin users: two of five in the omeprazole group and one of four in the control group developed PUD. Dialysis patients in the omeprazole group had fewer requirements of blood transfusion than the control group patients (2% vs. 11.1%, p = 0.009). Although mean hematocrit levels in both groups were not statistically different, patients in the omeprazole group received lower erythropoietin (EPO) doses than those in the control group while achieving similar hematocrit levels (1469 ± 287 vs. 1904 ± 398 units, p = 0.024). There was no difference in the percentage of patients receiving iron supplementation between the two groups (12/49 vs. 10/36, p > 0.05). Kaplan–Meier analysis revealed a higher cumulative ulcer rate in the control group (, p = 0.04). Nevertheless, we found two patients with GI bleeding diagnosed as having colonic angiodysplasia, instead of gastric or duodenal lesions.
Table 2. Comparison of outcomes between the omeprazole and control groups
Assessment of Safety and Side Effects
During the study period, there were no serious adverse events in the omeprazole group. One patient in this group prematurely discontinued the study due to palpitation. There was no significant change in body weight before and after omeprazole treatment (57.6 ± 9.4 vs. 58.6 ± 10.0 kg, p > 0.05). In the omeprazole group, one 45-year-old female with secondary hyperparathyroidism and one 78-year-old female with severe osteoporosis had femoral neck fractures because of accidental falls during the study period.
DISCUSSION
This prospective study was conducted to investigate the effects of prophylactic omeprazole on the occurrence of PUD in dialysis patients. Our results demonstrated that prophylactic omeprazole use not only reduced the risk of PUD but also decreased the requirement for blood transfusion and EPO doses among MHD patients. Furthermore, our study revealed the cost-effectiveness of omeprazole use, particularly with respect to the expense of EPO as well as hospitalization for bleeding complications. However, this efficacy was not seen in dialysis patients on regular aspirin or warfarin.
In the past, PUD with its high rate of morbidity and mortality was a major threat to the general population.Citation13 With the advance in medical therapies and the discovery of H. pylori, PUD outcomes have greatly improved.Citation7 However, despite great progress in endoscopic and pharmacologic treatments, a high risk for PUD-related bleeding complications still exists in the dialysis population.Citation3,Citation4,Citation14,Citation15 The recurrence rate of PUD has been reported to be significantly higher in dialysis patients than in those with normal kidney function.Citation4 Frequent occurrence of PUD in long-term dialysis patients not only affects the quality of life but also decreases the levels of hematocrit, a predictor of morbidity and mortality in these patients.Citation16 Therefore, the development of strategies to lower the incidence and severity of PUD is important in clinical practice. As eradication of H. pylori did not prevent PUD recurrence in end-stage renal disease patients,Citation4 other methods to reduce ulcer rates need to be developed. Our results indicate that prophylactic omeprazole use might be an effective strategy for preventing PUD among MHD patients.
One interesting finding of our study was that prophylactic omeprazole had no effect on the ulcer rate of MHD patients with regular aspirin or warfarin use. This result has several interpretations. First, most of our patients with aspirin or warfarin use were >60 years of age and had multiple comorbid conditions. Holden et al.Citation2 reported the hazard ratio for the first major bleeding event to be 3.59 for warfarin exposure and 5.24 for aspirin exposure, with respect to MHD patients without warfarin or aspirin use. Hence, an intervention involving PPIs alone might not change the incidence of PUD in this very high risk group. Second, the small patient size limited the statistical power, which implies that the results of larger-scale studies may differ. Finally, some patients on aspirin or warfarin in both groups would intermittently stop it if they felt abdominal discomforts during the study period, which might explain why we did not find a significant effect of prophylactic omeprazole on the incidence of PUD in these patients.
During the study period, we did not notice serious adverse events associated with omeprazole use. Although long-term use of PPI has been shown to lead to body weight gain in the general population by relieving the symptoms of PUD and increasing appetite,Citation17 our result was not consistent with this finding. It is possible that the limited water intake as well as diet control in the dialysis population minimized this undesired effect. Another concern regarding chronic omeprazole use among MHD patients is its effect on bone metabolism. Omeprazole has been shown to impair gastric acid secretion and have a negative influence on calcium homeostasis and bone mass, thus increasing the risk of fracture in the general population.Citation18 Kirkpantur et al.Citation19 also indicated that PPI therapy might be associated with lower serum calcium levels, higher intact PTH levels, and lower bone mineral density among MHD patients. However, our study did not find any differences in serum calcium, phosphorus, and intact PTH levels between the two groups. Even though we observed that two postmenopausal women in the omeprazole group had events of traumatic femoral neck fracture during the study period, no statistically significant increase in the risk of bone fracture was observed in our study. We propose two factors to account for the discrepancies between previous reports and our results. First, a complex relationship exists among calcium, phosphorus, vitamin D, parathyroid hormone, and bone mineral density, especially in the dialysis population. Further, bone fracture in the dialysis population could be caused by metabolic bone disease, senile osteoporosis, or other factors, despite lack of PPI use.Citation20 Thus, the association between bone fracture and PPI use in patients undergoing MHD remains unclear. Second, we strictly controlled serum calcium, phosphorus, and parathyroid hormone levels according to K-DOQI guidelines,Citation21 which possibly decreased the effects of omeprazole on bone metabolism.
Our study has several limitations. First, we did not perform periodic endoscopic examinations on all subjects, which might underestimate peptic ulcer rates in both groups. Second, most enrolled subjects in our study had several risk factors of PUD, particularly old age and coexisting medical conditions.Citation7 It is uncertain whether prophylactic omeprazole would have similar effects on young dialysis patients without multiple comorbidities. Third, we did not measure serum 25-OH vitamin D levels or bone mineral density to investigate changes in bone metabolism among patients receiving omeprazole. Finally, our study was limited to a small number of patients and a single center. Further prospective, multicenter, large-scale controlled trials should be carried out to confirm our findings.
In conclusion, the prophylactic use of omeprazole in MHD patients might lower the incidence of PUD and reduce treatment cost of these patients. However, our study did not demonstrate its efficacy in MHD patients on regular aspirin or warfarin. Furthermore, its safety should be assessed by longer-term and larger-scale controlled studies.
ACKNOWLEDGMENTS
We appreciate the effort of all dialysis staff at Zen-Ho Dialysis Center for collection of clinical data and administration of questionnaires. We also thank Giselle Yang for secretarial assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Toke AB. GI bleeding risk in patients undergoing dialysis. Gastrointest Endosc. 2010;71:50–52.
- Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–110.
- Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44–49.
- Tseng GY, Lin HJ, Fang CT, . Recurrence of peptic ulcer in uraemic and non-uraemic patients after Helicobacter pylori eradication: A 2-year study. Aliment Pharmacol Ther. 2007; 26:925–933.
- Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96–103.
- Nakajima F, Sakaguchi M, Amemoto K, Helicobacter pylori in patients receiving long-term dialysis. Am J Nephrol. 2002;22:468–472.
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461.
- Chan FK, Hung LC, Suen BY, Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104–2110.
- Chan FK, Ching JY, Hung LC, Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352:238–244.
- Yeomans N, Lanas A, Labenz J, Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am J Gastroenterol. 2008;103:2465–2473.
- Howden CW, Payton CD, Meredith PA, Antisecretory effect and oral pharmacokinetics of omeprazole in patients with chronic renal failure. Eur J Clin Pharmacol. 1985;28:637–640.
- Yen TH, Lin JL, Lin-Tan DT, Hsu CW, Weng CH, Chen YH. Spectrum of corrosive esophageal injury after intentional paraquat ingestion. Am J Emerg Med. 2010; 28:728–733.
- Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal hemorrhage. Gut. 1996;38:316–321.
- Tseng GY, Lin HJ. Aspirin prescription and outcomes in hemodialysis patients. Am J Kidney Dis. 2008;51:1070–1071.
- Tseng GY, Lin HJ. Factors aside from Helicobacter pylori play an important role in the occurrence of peptic ulcer in dialysis patients. Kidney Int. 2009;75:1114–1115.
- Robinson BM, Joffe MM, Berns JS, Pisoni RL, Port FK, Feldman HI. Anemia and mortality in hemodialysis patients: Accounting for morbidity and treatment variables updated over time. Kidney Int. 2005;68:2323–2330.
- Yoshikawa I, Nagato M, Yamasaki M, Kume K, Otsuki M. Long-term treatment with proton pump inhibitor is associated with undesired weight gain. World J Gastroenterol. 2009; 15:4794–4798.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953.
- Kirkpantur A, Altun B, Arici M, Turgan C. Proton pump inhibitor omeprazole use is associated with low bone mineral density in maintenance hemodialysis patients. Int J Clin Pract. 2009;63:261–268.
- Toussaint ND, Elder GJ, Kerr PG. A rational guide to reducing fracture risk in dialysis patients. Semin Dial. 2010; 23:43–54.
- K/DOQI. Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2003;42(4 Suppl. 3): S1–S201.