Abstract
Background: Oxidative stress has been implicated in the cardiovascular complications that affect hemodialysis (HD) patients. Ethylene-vinyl alcohol copolymer (EVAL) dialyzer membrane induces less production of reactive oxygen species as compared to conventional dialyzers. We evaluated the impact of EVAL membrane on plasma protein oxidation in HD patients. Methods: HD patients treated with cellulose triacetate (CTA) dialyzers were selected. In the first study performed in a 2-month crossover design alternating between CTA and EVAL, nonmercaptalbumin and advanced oxidation protein products levels were measured in the predialysis blood from 10 subjects. In the second study, predialysis plasma myeloperoxidase levels were measured before and after a 2-week EVAL treatment on 12 patients. Results: Plasma advanced oxidation protein products levels were reduced after a 2-month EVAL treatment and increased again after CTA treatment, although the nonmercaptalbumin proportions were not affected significantly by the change in dialyzer membranes. The following study, a 2-week EVAL treatment, showed the decrease in myeloperoxidase levels immediately before HD. Conclusion: The frequent use of EVAL dialyzers has been shown to reduce protein oxidation, possibly through the suppression of circulating phagocytes. This novel biocompatible dialyzer is expected to protect cardiovascular mortality in HD patients.
INTRODUCTION
Cardiovascular disease is the leading cause of mortality in hemodialysis (HD) patients.Citation1 Numerous studies on uremic patients have suggested that inflammation and malnutrition are significant predictors for cardiovascular mortality, rather than traditional risk factors such as hypertension and hypercholesterolemia.Citation2–5 However, definite pathophysiologic relationships between inflammation/malnutrition and cardiovascular morbidity remain to be elucidated, and oxidative stress is possibly a major factor that mediates these relationships in HD patients.Citation4,Citation6
Oxidant-derived tissue injury occurs when the production of oxidants exceeds local antioxidant capacity. Extracellular fluids contain various low-molecular-weight antioxidants (ascorbate, etc.) and antioxidant enzymes such as glutathione peroxidase and superoxide dismutase. The albumin molecule, which has a cysteine residue with a free thiol group, is the most abundant antioxidant in plasma.Citation7 Therefore, a lower albumin concentration may not only reflect a poorer nutritional condition of the HD patients but could also contribute to cardiovascular events. Recently, oxidant forms of albumin, such as advanced glycation end products-modified albumin and advanced oxidation protein products (AOPP), have been demonstrated to be determinants of oxidative stress in uremia.Citation8,Citation9 Alterations in the chemical state as well as in the amounts of albumin seem to affect their redox state via a lower antioxidant capacity in HD patients.
Thus far, several investigators have demonstrated that the HD procedure can improve the redox balance toward normal through a single dialysis session.Citation10,Citation11 These results are likely to be logical if the uremic environments are characterized by an alteration in the redox state. An earlier paper showing the presence of a low-molecular-weight dialyzable oxidant in uremic plasmaCitation12 also supports this possibility. In the HD procedure, however, various biological reactions can occur during the contact between artificial materials and blood components, leading to potential pro-inflammatory pro-oxidative effects.Citation13 Gawaz et al.Citation14 reported that HD with conventional dialyzers induced the production of reactive oxygen species (ROS) resulting from neutrophil–platelet aggregation via the upregulation of adhesion molecules. Interestingly, no significant changes in ROS production by the neutrophils have been revealed during HD using ethylene-vinyl alcohol copolymer (EVAL) dialyzers,Citation15 which are able to eliminate high-molecular-weight molecules because of larger pores when compared with conventional dialyzers.Citation16
In the current study, to assess the impact of EVAL dialyzers on the plasma levels of oxidative protein, we investigated whether treatment with EVAL dialyzers can induce a decrease in nonmercaptalbumin (HNA) and AOPP levels in HD patients who had been treated with cellulosic dialyzers. The levels of myeloperoxidase (MPO), which catalyzes the reaction for some oxidative forms, were also measured. Our results suggested the possibility that the frequent use of a kind of biocompatible dialyzer reduces oxidative stress in HD patients.
METHODS
Patients
Uremic patients aged >50 years, undergoing 4-h-long HD thrice a week for at least 1 year, and using cellulose triacetate (CTA) dialyzer membranes for at least 3 months were recruited from the Ohtemachi clinic. Ten patients (seven men and three women) aged 59–82 years with a duration of HD ranging from 1 to 8 years were enrolled in the study for evaluating oxidative protein. In the following study for MPO evaluation, 12 different patients (6 men and 6 women) aged 61–83 years with duration of HD ranging from 1 to 16 years were enrolled. The renal failure of each patient was well controlled by the HD treatment. They were taking standard medications, including vitamin D3, calcium carbonate, antihypertensive agents, and erythropoietin. None of the patients had unstable vascular access, hepatic failure, or any life-threatening diseases. Written informed consent was obtained from all patients. This study was approved by the ethics committee of the clinic (No. 200701 and 200801). The profiles of our patients are summarized in .
Table 1. Clinical characteristics and baseline laboratory values in patients recruited in the first and second studies
Study Design
To evaluate the impact of EVAL dialyzers we treated the patients who had been treated with CTA dialyzers with EVAL dialyzers. The dialyzers we selected were FB-190P produced by NIPRO (Osaka, Japan), which belongs to CTA membranes, and KF-20 produced by Asahi Kasei Kuraray Medical (Tokyo, Japan), which belongs to EVAL membranes. Both have nearly the same membrane areas. The first study was performed in a 2-month crossover design. In the 10 patients using FB-190P, their dialyzers were changed to KF-20 for 2 months and then changed back to FB-190P. The average urea reduction rates in the period during which KF-20 was used were slightly lower than those in the period during which FB-190P was used (67.5% vs. 71.4%). At three time points (before changing to KF-20, 2 months after the change, and 2 months after the change back to FB-190P), blood was drawn from these patients immediately before the first dialysis session of the week. The blood samples were taken into a tube containing EDTA and 40 µg/mL of butylhydroxytoluene as an antioxidant, and plasma samples were separated by centrifugation and stored at –80°C until analysis. In the second study to examine MPO levels, the dialyzers of the 12 patients using FB-190P were changed to KF-20 for 2 weeks. Blood samples immediately before the first dialysis session of the week were collected with a heparinized syringe from each patient before the change to KF-20 and 2 weeks after the change, and plasma samples were stored until analysis. Pre-enrolment medications that might affect plasma redox balance, such as angiotensin II receptor antagonists and statins, were kept unchanged from 3 months prior to the initiation to the end of the study. Furthermore, any supplements such as vitamins, l-carnitine, and coenzyme Q10 were not administered for the duration of the study. HD prescriptions, including the composition of dialysates, blood flow rates, and dialysate flow rates, were not changed.
Nonmercaptalbumin Measurement
Plasma levels of mercaptalbumin (HMA) and HNA were measured according to the method described previously,Citation17 with high-performance liquid chromatography (HPLC). The chromatograph was equipped with a fluorescence detection system that consisted of a model 717plus auto sampler, model 600 double-plunger pump, and model 2475 fluorescence detector (all from Waters, Tokyo, Japan). A Shodex-Asahipak ES-502N column (10 × 0.75 cm inner diameter, DEAE-form for ion-exchange HPLC; Showa Denko Co., Tokyo, Japan; column temperature, 35 ± 0.5°C) was used in this study. Elution was performed by linear gradient elution with increasing ethanol concentrations from 0% to 7.5% for 40 min in 0.05 mol/L sodium acetate and 0.40 mol/L sodium sulfate mixture (pH 4.85) at a flow rate of 1.0 mL/min. From HPLC profiles of plasma albumin, the values of HMA and HNA fraction were evaluated by dividing the area under the peak corresponding to each fraction by total area of plasma albumin.
AOPP Measurement
Plasma levels of AOPP were measured according to the method described by Witko-Sarsat et al.Citation18 Briefly, AOPP levels were measured spectrophotometrically by a microplate reader and calibrated with chloramine-T (Wako, Osaka, Japan) solutions, which in the presence of potassium iodide absorb at 340 nm. In test wells, 200 µL of plasma diluted 1:1.5 in phosphate-buffered saline was placed on a 96-well microtiter plate and 20 µL of acetic acid was added. In standard wells, 10 µL of 1.16 mol/L potassium iodide was added to 200 µL of chloramine-T solutions (0–100 µmol/L) followed by 20 µL of acetic acid. The absorbance of the reaction mixture is immediately read at 340 nm on the microplate reader against a blank containing 200 µL of phosphate-buffered saline, 10 µL of potassium iodide, and 20 µL of acetic acid. Because chloramine-T absorbance at 340 nm is linear within the range of 0–100 µmol/L, AOPP concentrations are expressed in micromoles per liter of chloramine-T equivalents. The intra-assay CV of our method for AOPP was 5.0% and the inter-assay CV was 9.3%.
MPO Measurement
A sandwich-based enzyme-linked immunosorbent assay (Myeloperoxidase ELISA Kit, Biocheck, Foster City, CA, USA) was used to measure plasma MPO level with the range between 0.5 and –40 ng/mL. Laboratory technicians were blinded to patient identifiers and clinical parameters.
Statistical Analysis
The results are expressed as the mean (SD). Student's t- was used to evaluate the statistical significance for the paired data. Values with a p-value <0.05 were considered statistically significant.
RESULTS
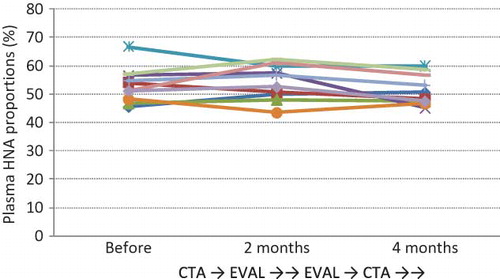
Plasma Predialysis HNA Proportions Did Not Improve with 2 Months of Treatment with EVAL Membranes
HPLC of human serum albumin provided a clear resolution of HNA and HMA, which are the oxidized and reduced forms of human serum albumin, respectively. HNA has a reversibly oxidized form (HNA-1) and a strongly oxidized form (HNA-2), which accounts for a very small fraction of the total HNA. Although total HNA constitutes 20–25% of plasma albumin in healthy subjects,Citation19,Citation20 our patients showed significantly higher percentages of HNA (HNA-1 and HNA-2) on starting HD; this result is similar to the findings of previous reports.Citation17,Citation20 In this crossover study, however, there were no significant differences between the results before and after EVAL treatment and between the results before and after CTA treatment (). EVAL treatment for the 2-month period demonstrated no advantage in terms of HNA reduction.
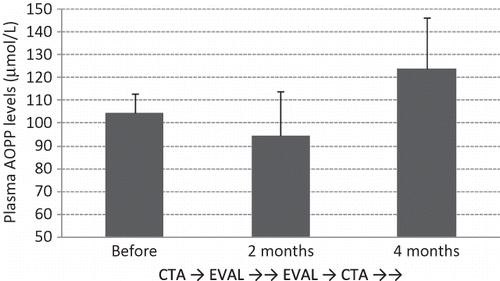
Plasma Predialysis AOPP Levels Were Reduced with 2 Months of EVAL Treatment
Our patients showed a marked increase in baseline AOPP levels as compared to healthy controls (data not shown); this result is consistent with the findings of a previous paper.Citation18 As shown in , predialysis AOPP levels decreased after the 2-month EVAL treatment, and then increased after 2 months of CTA treatment. The average AOPP levels were 104.6, 94.6, and 124.1 µmol/L before EVAL, after EVAL (and before CTA), and after CTA treatment, respectively. A significant difference (p < 0.001) was noted between the results before and after CTA treatment, but not between the results before and after EVAL treatment (p = 0.070); however, the AOPP levels did decrease to a certain extent after EVAL treatment.
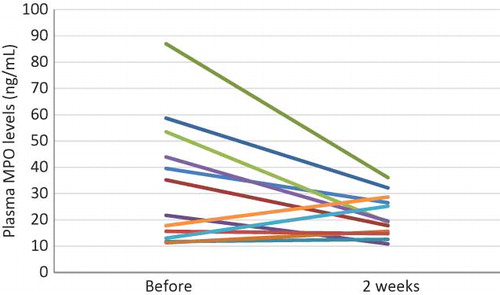
Plasma MPO Levels Were Significantly Reduced by Short-Term Use of EVAL Dialyzers
Because AOPP production is partly catalyzed by MPO, we determined the plasma MPO levels before and after the use of the EVAL dialyzer. As shown in , with the use of EVAL membranes for only 2 weeks, there was a significant decrease in the plasma MPO levels recorded immediately before HD (34.2 ± 23.6 ng/mL vs. 21.7 ± 8.0 ng/mL). In the case of seven patients whose MPO levels were >30 ng/mL when the CTA membranes were used, the use of EVAL dialyzers dramatically decreased the MPO levels (53.1 ± 18.8 ng/mL vs. 25.4 ± 7.7 ng/mL, p = 0.002).
DISCUSSION
It has been well recognized that human serum albumin is quite vulnerable to ROS.Citation21 Oxidation of tyrosine residues of albumin, leading to the formation of dityrosine, protein aggregation, cross-linking, and fragmentation, is an example of ROS-mediated protein damage in vitro.Citation22 In a uremic environment, plasma albumin is continuously exposed to oxidative stress; as a result, the conformation and function of albumin may be altered. Witko-Sarsat et al. isolated and characterized dityrosine-containing protein cross-linking products in the plasma of HD patients, which they designated AOPP.Citation18 Their following work revealed that AOPP-human serum albumin prepared in vitro was capable of triggering the oxidative burst of human monocytes, indicating that AOPP act as a mediator of oxidative stress and monocyte respiratory burst.Citation9
Our crossover study demonstrated the possibility that a biocompatible dialyzer can lead to the suppression of plasma AOPP levels. Decreased AOPP levels may not only result from a decline of oxidative stress and inflammation but may also contribute to it. Another study showed that predialysis MPO levels decreased after short-term use of the biocompatible dialyzer. As MPO is secreted by activated phagocytes during inflammation, the decrease in circulatory MPO levels may indicate the reduction in phagocyte activation.Citation23 Moreover, MPO is principally able to catalyze the production of hypochlorous acid, a powerful oxidant derived from the chloride ion and hydrogen peroxide. Considering that the oxidation products of tyrosine residues, such as 3-chlorotyrosine and dityrosine, are generated by an MPO-catalyzed reaction,Citation24,Citation25 the decrease in AOPP levels can be accounted for by the suppression of oxidant production via the decrease in MPO levels.
What are the reasons for EVAL membranes to be distinct from CTA membranes in their influence on plasma AOPP levels? First, biochemical reactivity to the circulating cells of EVAL may be less than the reactivity to those of CTA. Sirolli et al.Citation15 demonstrated that adhesion receptor expression on neutrophils and monocytes was upregulated and that activated platelets increased in cellulose diacetate membranes as compared to in EVAL, resulting in an augmentation of hydrogen peroxide production via platelet–neutrophil co-aggregation. The downregulation of adhesion molecules in phagocytes during EVAL treatment also supports our results of suppressed MPO secretion. Collectively, EVAL treatment, as compared to CTA treatment, can suppress hydrogen peroxide production by a reduced reaction to circulating phagocytes.
The second point is whether plasma AOPP are dialyzed more through EVAL membranes than through CTA membranes. It is well known that EVAL membranes show a wide clearance spectrum from urea to high-molecular-weight materials more than β2-microglobulin.Citation16 Recently, we have revealed significantly higher albumin levels in whole dialysate drainage from EVAL than from CTA (2.1 vs. 0.7 g/session, unpublished data). The fibers of EVAL comprise hydrophilic vinyl alcohol and hydrophobic ethylene monomeric units. Their smooth surface and hydrophilic nature were demonstrated by atomic force microscopy and X-ray photoelectron spectroscopy, respectively.Citation26 These are related to less protein adsorption onto membranes.Citation26 Mera et al.Citation27 observed a slight decrease in α-helical content accompanied by a tertiary conformational change in albumin from HD patients. Taken together, there is a possibility that EVAL membranes eliminate the albumin fraction altered in the conformation as well as biological properties such as AOPP in a favorable way.
In HNA from predialysis plasma, however, changes similar to those in AOPP levels were not observed in our 2-month crossover study. Human serum albumin is composed of 585 amino acids, and cysteine residue at position 34 from the N-terminus has a mercapto group (SH group). The major HNA is the disulfide bonding form, which reversibly oxidizes the mercapto group by either cysteine or glutathione, according to the level of the surrounding oxidative stress.Citation19 In earlier reports, the HNA fraction of serum albumin decreased transiently in all HD patients after a single HD session, which was demonstrated by HPLC profiles.Citation19,Citation28 Furthermore, a recent study on peritoneal dialysis patients showed that their HNA proportions correlated significantly with serum urea nitrogen but not with other variables considered.Citation29 Taken together, HNA seems to be an unsettled albumin fraction that is influenced rigorously by certain dialyzable oxidants. Cystine or homocysteine–cysteine mixed disulfide, which was significantly increased in uremic patients and was decreased by HD,Citation30,Citation31 may play a role in the HNA–HMA conversion. The reversibility of HNA seems to have led to results inconsistent with AOPP, although the 2-month period in EVAL treatment may not be long enough to improve the baseline HNA levels.
Thus far, there have been several new therapeutic approaches designed to ameliorate the devastating consequences of cardiovascular disease through breaking antioxidants in this patient group. The risks and benefits of ascorbate administration in uremic patients will have to be weighed up carefully.Citation32,Citation33 In a recent study that evaluated secondary prevention with vitamin E for cardiovascular disease in uremic patients, there was no improvement in overall survival despite a marked protection in cardiovascular morbidity.Citation34 This evidence suggests the need to investigate additional strategies to improve cardiovascular morbidity and mortality. Our group has recently demonstrated that EVAL membrane is superior to polysulfone and vitamin E-bonded cellulose membranes for cutaneous microcirculation.Citation35 An approach with a new HD prescription, such as a biocompatible dialyzer, may become a candidate for further clinical trials.
In conclusion, we found that a 2-month treatment of biocompatible EVAL dialyzers decreased plasma AOPP levels, which is a novel marker of oxidative stress. Moreover, MPO levels in predialysis plasma were significantly decreased after EVAL treatment for only 2 weeks. Aside from the possibility of dominant AOPP removal through the EVAL membrane, the evidence suggests that frequent use of EVAL dialyzers reduces oxidative stress via the suppression of phagocyte activation. We are now analyzing the oxidation products of tyrosine residues in whole dialysate drainage. Furthermore, an investigation is needed to define whether EVAL dialyzers contribute to improving cardiovascular mortality in uremic patients.
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl. 3):S112–S119.
- Stenvinkel P, Heimbürger O, Paultre F, Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–1911.
- Suliman ME, Qureshi AR, Bárány P, Hyperhomocysteinemia, nutritional status, and cardiovascular disease in hemodialysis patients. Kidney Int. 2000;57(4):1727–1735.
- Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: A hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. 1997;8(3):475–486.
- Cheung AK, Sarnak MJ, Yan G, Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58(1):353–362.
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5): 1524–1538.
- Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. J Parenter Enteral Nutr. 1991;15(4):476–483.
- Mera K, Anraku M, Kitamura K, Oxidation and carboxy methyl lysine-modification of albumin: Possible involvement in the progression of oxidative stress in hemodialysis patients. Hypertens Res. 2005;28(12):973–980.
- Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161(5):2524–2532.
- Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58(6):2571–2578.
- Himmelfarb J, McMenamin E, McMonagle E. Plasma aminothiol oxidation in chronic hemodialysis patients. Kidney Int. 2002;61(2):705–716.
- Roselaar SE, Nazhat NB, Winyard PG, Jones P, Cunningham J, Blake DR. Detection of oxidants in uremic plasma by electron spin resonance spectroscopy. Kidney Int. 1995;48(1): 199–206.
- Tetta C, Biasioli S, Schiavon R, An overview of hemodialysis and oxidant stress. Blood Purif. 1999;17(2–3):118–126.
- Gawaz MP, Mujais SK, Schmidt B, Gurland HJ. Platelet-leukocyte aggregation during hemodialysis. Kidney Int. 1994;46(2):489–495.
- Sirolli V, Ballone E, Di Stante S, Amoroso L, Bonomini M. Cell activation and cellular-cellular interactions during hemodialysis: Effect of dialyzer membrane. Int J Artif Organs. 2002;25(6):529–537.
- Akiba T, Shibamoto T, Yamada T, Dialyzer clearance for dextran by high performance liquid chromatography. Jpn J Artif Organs. 1989;18(3):1232–1235.
- Soejima A, Kaneda F, Manno S, Useful markers for detecting decreased serum antioxidant activity in hemodialysis patients. Am J Kidney Dis. 2002;39(5):1040–1046.
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313.
- Sogami M, Era S, Nagaoka S, HPLC-studies on nonmercapt-mercapt conversion of human serum albumin. Int J Pept Protein Res. 1985;25(4):398–402.
- Terawaki H, Yoshimura K, Hasegawa T, Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004;66(5):1988–1993.
- Davies KJ. Protein damage and degradation by oxygen radicals I. General aspects. J Biol Chem. 1987;262(20):9895–9901.
- Heinecke JW, Li W, Daehnke HL3rd, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993;268(6):4069–4077.
- Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48(1):59–68.
- Himmelfarb J, McMenamin ME, Loseto G, Heinecke JW. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic Biol Med. 2001;31(10):1163–1169.
- Heinecke JW, Li W, Francis GA, Goldstein JA. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91(6):2866–2872.
- Bonomini M, Pavone B, Sirolli V, Proteomics characterization of protein adsorption onto hemodialysis membranes. J Proteome Res. 2006;5(10):2666–2674.
- Mera K, Anraku M, Kitamura K, Nakajou K, Maruyama T, Otagiri M. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem Biophys Res Commun. 2005;334(4): 1322–1328.
- Soejima A, Matsuzawa N, Hayashi T, Alteration of redox state of human serum albumin before and after hemodialysis. Blood Purif. 2004;22(6):525–529. Epub, December 3, 2004.
- Terawaki H, Matsuyama Y, Era S, Elevated oxidative stress measured as albumin redox state in continuous ambulatory peritoneal dialysis patients correlates with small uraemic solutes. Nephrol Dial Transplant. 2007;22(3):968.
- Robins AJ, Milewczyk BK, Booth EM, Mallick NP. Plasma amino acid abnormalities in chronic renal failure. Clin Chim Acta. 1972;42(1):215–217.
- Wilcken DE, Gupta VJ, Reddy SG. Accumulation of sulphur-containing amino acids including cysteine-homocysteine in patients on maintenance hemodialysis. Clin Sci (Lond). 1980;58(5):427–430.
- Halliwell B. Vitamin C: Antioxidant or pro-oxidant in vivo? Free Radic Res. 1996;25(5):439–454.
- Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292(5524):2083–2086.
- Boaz M, Smetana S, Weinstein T, Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet. 2000;356(9237):1213–1218.
- Sato M, Morita H, Ema H, Yamaguchi S, Amano I. Effect of different dialyzer membranes on cutaneous microcirculation during hemodialysis. Clin Nephrol. 2006;66(6):426–432.