Abstract
Studies indicate that the antihyperglycemic effects of Syzygium aromaticum-derived oleanolic acid (OA) are mediated in part through increased hepatic glycogen synthesis. Accordingly, this study assessed the influence of OA on the activity of glucokinase (GK) and hexokinase (HK) of skeletal muscle and liver tissues in streptozotocin (STZ)-induced diabetic rats. After 5 weeks of OA treatment, hepatic and gastrocnemius muscle glycogen concentrations and activities of GK and HK were measured spectrophotometrically in reactions where the oxidation of glucose-6-phosphate (G-6-PDH) formed was coupled to nicotinamide adenine dinucleotide phosphate (NADP+) reduction catalyzed by G-6-PDH dehydrogenase. Rats treated with deionized water or standard hypoglycemic drugs acted as untreated and treated positive controls, respectively. STZ-induced diabetic rats exhibited depleted glycogen levels and low activities of glycogenic enzymes in muscle and hepatic tissues. OA administration restored these biochemical alterations to near normalcy. The combination of OA and insulin did not significantly alter the activities of HK and GK of STZ-induced diabetic rats, suggesting that glycogen synthesis can also occur from precursors such as amino acids or fructose and lactate. The attenuation of the activities of glycogenic enzymes with concomitant increases of hepatic and muscle glycogen concentrations of STZ-induced diabetic rats provides a therapeutic strategy for diabetes treatment.
INTRODUCTION
Blood glucose homeostasis is mainly maintained by the conversion of glucose to glycogen in the skeletal muscles and liver with the latter playing a pivotal role in buffering postprandial hyperglycemia in mammals.Citation1 The importance of liver and skeletal muscles in glucose homoeostasis is supported by a correlation between depleted liver and muscle glycogen concentrations in diabetes and decreased activities of insulin-stimulated glycogenic enzymes, [glucokinase (GK), hexokinase (HK), and glycogen synthase].Citation1–3 Indeed, depleted glycogen concentration in chronic insulin-dependent diabetes mellitus has been ascribed to decreases in activities of insulin-dependent and insulin-sensitive HKs and glycogen synthase.Citation1–3 The HK isoform family of enzymes phosphorylates glucose to glucose-6-phosphate (G-6-PDH) for further utilization in cells and for glycogen synthesis in hepatic and muscle tissues.Citation4 GK (HK-IV) is the predominant glucose-phosphorylating enzyme in hepatocytes, pancreatic β cells, and glucagon-secreting cells of the pancreasCitation5 whereas two isoforms of HK, I and II, are found in the muscles.Citation6 As insulin treatment normalizes glycogenic enzymes in diabetes mellitus,Citation7 therapeutic agents that directly or indirectly activate these enzymes have potential for glycemic control in diabetes. Although several options are currently available for the treatment of diabetes, no single marketed drug is capable of achieving long-lasting blood glucose control in the majority of patients or compensating for metabolic derangements.Citation8,Citation9 We have shown that the hypoglycemic effect of the triterpene oleanolic acid (OA) in streptozotocin (STZ)-induced diabetic rats is in part mediated through increased hepatic glycogen synthesis.Citation10,Citation11
Guided by this fundamental observation and further evidence that indicates that the plant-derived triterpene ursolic acid (UA) attenuates hyperglycemia as well as lipid metabolism in STZ-induced diabetic animals by improving the polyol pathway,Citation12 we speculated that OA increases glycogen concentration of diabetic rats by modulating carbohydrate-metabolizing enzymes. Accordingly, this study was designed to assess the effects of OA on the activity of key glycogenic enzymes, GK and HK, in skeletal muscle and liver tissues of STZ-induced diabetic rats to further elucidate the mechanism(s) of hypoglycemic effects of the triterpene. The activity of these key enzymes of glucose metabolism has been shown to constitute a valuable tool to modulate the capacity of the cell to take up and utilize glucose in various cell types.Citation12
MATERIALS AND METHODS
Drugs and Chemicals
Drugs were sourced from standard pharmaceutical suppliers. All other chemicals used were purchased from standard commercial suppliers and were of analytical grade quality.
Isolation of OA
OA was isolated from Syzygium aromaticum [(Linnaeus) Merrill and Perry] (Myrtaceae) (cloves) flower buds using a standard protocol that has been validated in our laboratory.Citation11,Citation13 Briefly, air-dried powdered flower buds of S. aromaticum (1.74 kg) were sequentially extracted thrice at 24 h intervals with 3 L of hexane, dichloromethane, ethyl acetate, and methanol on each occasion. Recrystallization of ethyl acetate solubles (25.5 g) that contained mixtures of OA/UA and methyl maslinate/methyl corosolate with ethanol yielded pure OA whose structure was confirmed by spectroscopic analysis using 1D and 2D, 1H and 13C nuclear magnetic resonance techniques. Preliminary studies indicated that the hypoglycemic effects of S. aromaticum-isolated OA and commercial OA were similar and hence the plant-extracted OA was used in the experiments as it is less costly.
Animals
Male Sprague–Dawley rats (250–300 g body weight) bred and maintained at Biomedical Research Unit, University of KwaZulu-Natal, were used in this study. The animals had free access to standard rat chow (Meadows, Pietermaritzburg, South Africa) and water, with a 12 h light/12 h dark cycle. Procedures involving animals and their care were conducted in conformity with institutional guidelines of the University of KwaZulu-Natal.
Induction of Diabetes Mellitus
Diabetes mellitus was induced in male Sprague–Dawley rats with a single intraperitoneal injection of STZ (60 mg kg−1) dissolved in freshly prepared 0.1 M citrate buffer (pH 6.3). Animals that exhibited glucosuria after 24 h, tested by urine strips (Rapidmed Diagnostics, Sandton, South Africa), were considered diabetic. Blood glucose concentration of 20 mmol L−1 or above measured after 1 week was considered as a stable diabetic state before experimental procedures. Control animals were injected with the vehicle, citrate buffer.
Experimental Design
Nondiabetic and STZ-induced diabetic rats were divided into separate groups to study the short-term effects (5 weeks) of OA on GK and HK along with glycogen content of the skeletal muscle and liver tissues (n = 6 in each group).
Treatment
Separate groups of nondiabetic and STZ-induced diabetic rats housed individually in Makrolon polycarbonate metabolic cages (Techniplats, Labotec, Midrand, South Africa) at the Biomedical Resource Unit, University of KwaZulu-Natal, were orally administered with OA (80 mg kg−1, p.o.) twice every third day at 0900 and 1500 by means of a bulbed steel tube for 5 weeks. The dose was based on a previous work that was undertaken to establish and assess synergistic antihyperglycemic effects between plant-derived OA and insulin in STZ-induced diabetic rats.Citation11 Separate groups of nondiabetic and STZ-induced diabetic rats administered insulin (200 µg kg−1, s.c.) or metformin (500 mg kg−1, p.o.) acted as positive control animals. The influence of OA on insulin effects was studied in separate groups of animals that were treated with combined OA (80 mg kg−1) and insulin (200 µg kg−1, s.c.). Rats treated with deionized water (3 mL kg−1, p.o.) served as control animals.
Harvesting of Liver and Muscle Tissue
All animals were anesthetized with halothane after 5 weeks and samples of liver and gastrocnemius muscle (1–1.5 g) were harvested for the assessment of hepatic GK (EC 2.7.1.1) and gastrocnemius muscle HK (EC 2.7.1.1) content along with glycogen concentrations. Samples of liver and muscle tissue for glycogen determination were snap frozen in liquid nitrogen and stored at −80°C in a Bio Ultra freezer (Mallinckrodt, Cleveland, OH, USA) until required for analysis. Liver and muscle tissue samples (0.5–1 g) harvested for enzyme analyses were placed in ice-cold 50 mM Tris-HCl buffer containing 100 mM KCl and 1 mM EDTA, pH 8, before further processing for enzyme analysis.
Glycogen Content
The glycogen concentration was determined as previously described by Ong and KhooCitation14 with modifications from our laboratory.Citation15 Briefly, the tissue samples (1–1.5 g) were homogenized in 2 mL of 30% KOH solution and digested at 100°C for 30 min and then cooled in ice-saturated sodium sulfate. The glycogen was precipitated with ethanol and then pelleted, washed, and resolubilized in deionized water. Glycogen standards (10–2000 mg L−1) were also prepared using glycogen powder. The glycogen concentration was determined by its reaction with the anthrone reagent [2 g anthrone/1 of 95% (v/v) H2SO4] after which absorbance was measured at 620 nm using a Novaspec II spectrophotometer (Biochrom Ltd., Cambridge, UK).
Enzymatic Estimations
To assess whether OA influences enzymes of carbohydrate metabolism, we studied the effect of OA on key glycogenic enzymes in hepatic and gastrocnemius muscle tissues of nondiabetic and STZ-induced diabetic rats. Liver and muscle tissue samples were cut into small pieces and then washed once with ice-cold 50 mM Tris-HCl buffer containing 100 mM KCl, 1 mM EDTA, pH 8. The samples were homogenized in the same buffer using a ratio of 1 g tissue to 10 mL of buffer. Liver samples were homogenized with a motor-driven Potter-Elvehjem homogenizer (Glas-Col“, Terre Haute, IN, USA) at 4000 rpm (six passes) whereas muscle tissues were homogenized using an Ultra-Turrax homogenizer (IKA, Staufen, Germany) at 20,000 rpm (3 × 5 s bursts) allowing for cooling on ice in between homogenizations. The homogenates were then aliquoted into Eppendorf tubes and stored at −80°C until required for analysis. Before enzyme determination homogenates were thawed on ice, mixed gently, and then centrifuged at 10,000 × g for a minute in an MSE Micro Centaur microfuge (Pegasus Scientific Inc., Burtonsville, MD, USA). The resulting supernatant was used for enzyme assay.
Measurement of HK (EC 2. 7. 1. 1.) Activity
The activity of HK in the gastrocnemius muscle and liver tissues was determined as previously described by Goward et al.Citation16 with modifications. Briefly, the activity was measured by an assay in which the oxidation of G‐6-PDH produced is coupled to the reduction of nicotinamide adenine dinucleotide phosphate (NADP+) catalyzed by G-6-PDH dehydrogenase as shown in the equation below:
The reaction mixture volume (2.71 mL) contained the following: 20 mM triethanolamine-HCl buffer (pH 7.6), 222 mM glucose, 8 mM MgCl2, 0.91 mM NADP+ (Na salt), 0.64 mM ATP (Na salt), 5 mM mercaptoethanol, 0.55 U mL−1 G-6-PDH to which 100 µL of the gastrocnemius muscle or liver homogenate was added. The reaction that was carried out at 30°C in a water-jacketed spectrophotometer Varian Cary 50 Conc UV–Visible (SMM Instruments, Midrand, South Africa) was monitored for 10 min by following the appearance of reduced nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm.Citation16
Hepatic GK (EC 2. 7. 1. 2.) Activity
GK activity was measured by an assay in which the oxidation of G-6-PDH produced is coupled to the reduction of NADP+ catalyzed by G-6-PDH dehydrogenase as shown below:
The reaction mixture volume (2.71 mL) contained the same cocktail as for HK. GK activity was estimated by subtracting the rate of NADPH formation in the presence of 0.5 mM glucose (scoring low-Km HK activity) from that obtained in the presence of 222 mM glucose (scoring total HK and GK activity) as described by Salas et al.Citation17
Protein Determination
Protein was determined by the method of Lowry et al.Citation18 with 200 µg mL−1 bovine serum albumin (BSA) as the standard. Samples were diluted with 0.5 N NaOH and aliquots of the diluted samples made up to 0.5 mL with deionized water. The mixtures were incubated for 15 min at 40°C following the addition of 5 mL alkaline reagent (100 volumes 4% Na2CO3, 1 volume 4% CuSO4, and 4% KNaC4H4O6). Subsequently, 0.5 mL of Folin–Ciocalteu reagent (diluted ½ with deionized water) was added and color was allowed to develop for 30 min at room temperature. The absorbance was read at 600 nm using a Varian Cary 50 Conc UV–Visible spectrophotometer (SMM Instruments). A standard graph of 0–100 µg BSA in 0.5 mL deionized water was also prepared.
Statistical Analysis
All data were expressed as means ± standard error of means (SEM). Statistical comparison of the differences between the control means and experimental groups was performed with GraphPad InStat Software (version 4.00, GraphPad Software, San Diego, CA, USA), using one-way analysis of variance, followed by Tukey–Kramer multiple comparison test. A value of p < 0.05 was considered significant.
RESULTS
Effects of OA on Glycogen Concentrations
By the end of the 5-week experimental period, the hepatic glycogen concentration in STZ-induced diabetic rats (17.1 ± 1.9 mg 100 mg−1 tissue) was significantly (p < 0.05) low by comparison with control nondiabetic animals (28.4 ± 0.4 mg 100 mg−1 tissue) at the corresponding time. Similarly, the gastrocnemius glycogen concentration of STZ-induced rats (1.1 ± 0.1 mg−1 100 mg−1 tissue) was significantly (p < 0.05) depleted when compared with that of nondiabetic rats (2.6 ± 0.3 mg 100 mg−1 tissue). OA treatment of STZ-induced rats for 5 weeks restored the hepatic and gastrocnemius muscle glycogen concentrations to near normalcy (30.3 ± 0.6 mg 100 mg−1 tissue and 2.0 ± 0.4 mg 100 mg−1 tissue, respectively).
Insulin and metformin increased the glycogen concentration of hepatic and gastrocnemius muscle tissues of STZ-induced diabetic rats. By comparison to all groups of treatments, combined OA and insulin administration evoked the most pronounced glycogen concentration restoring activity in hepatic and gastrocnemius muscle tissues of STZ-induced diabetic rats (56.4 ± 0.5 mg 100 mg−1 tissue and 3.1 ± 0.3 mg 100 mg−1 tissue, respectively).
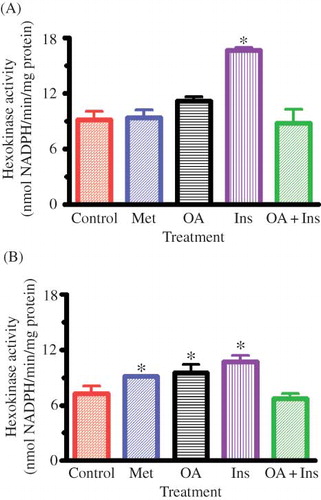
Muscle HK Activity
At the end of the 5-week experimental period, STZ-induced diabetic controls showed significant decrease in the activity values of gastrocnemius muscle HK by comparison with those of nondiabetic rats (cf. and B). Oral treatment with OA as well as insulin and metformin twice every third day for 5 weeks to STZ-induced diabetic rats significantly (p < 0.05) increased the gastrocnemius HK activity (). However, gastrocnemius HK activity of nondiabetic rats was only increased by insulin (). Combined OA and insulin or metformin treatments did not alter the gastrocnemius HK activity of both nondiabetic and STZ-induced diabetic rats.
Figure 1. Effects of OA, metformin (Met), insulin (Ins), and combined OA and insulin (OA + Ins) on gastrocnemius muscle HK activity in nondiabetic (A) and STZ-induced diabetic (B) rats. Values are presented as means, and vertical bars indicate SEM (n = 6 in each group).
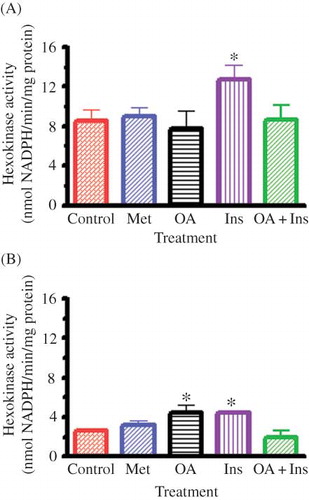
Hepatic HK Activity
shows that OA as well as insulin significantly (p < 0.05) increased hepatic HK activity of STZ-induced diabetic rats whereas metformin did not affect the activity. The hepatic HK activity of nondiabetic rats was increased only by insulin. Combined OA and insulin decreased hepatic HK activity of STZ-induced diabetic rats to levels that did not achieve statistical significance, but the activity in nondiabetic rats was not altered.
Figure 2. Effects of OA, metformin (Met), insulin (Ins), and combined OA and insulin (OA + Ins) on hepatic HK activity in nondiabetic (A) and STZ-induced diabetic (B) rats. Values are presented as means, and vertical bars indicate SEM (n = 6 in each group).
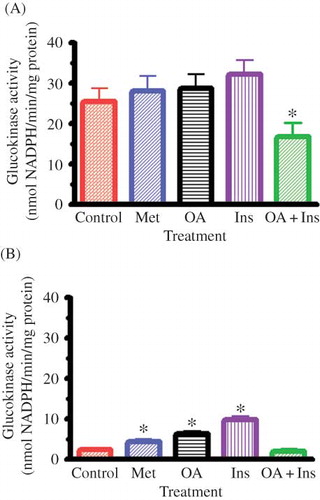
Hepatic GK Activity
OA as well as standard antidiabetic treatment significantly increased GK activity of STZ-induced diabetic rats, but the activity of nondiabetic rats was not altered by any treatment (). Combined OA and insulin administration, however, significantly inhibited hepatic GK activity of nondiabetic rats, but did not alter the activity of STZ-induced diabetic rats.
Figure 3. Effects of OA, metformin (Met), insulin (Ins), and combined OA and insulin (OA + Ins) on hepatic GK activity in nondiabetic (A) and STZ-induced diabetic (B) rats. Values are presented as means, and vertical bars indicate SEM (n = 6 in each group).
DISCUSSION
The results of this study extend our previous observations that OA elevates glycogen concentration of STZ-induced ratsCitation11 by showing that this effect is apparently mediated through increases in the activity of glycogenic enzymes. OA administration to STZ-induced diabetic rats resulted in significant increases of hepatic and muscle glycogen concentrations and the activity of HK and GK. These HK isoforms control glycogen synthase activation state, the enzyme that catalyzes the key step of glycogen synthesis through glucose phosphorylation. Interestingly, UA, an isomer of OA, has also been reported to upregulate glucose utilization and glycogen storage in experimental diabetic mice by modulating carbohydrate-metabolizing enzymes.Citation11
STZ-induced diabetic rats exhibited depleted hepatic and muscle glycogen levels and low activity of HK and GK perhaps due to low insulin levels caused by the destruction of pancreatic β cells by STZ.Citation19–24 The administration of OA to diabetic rats increased the activity of HK and GK with concomitant restoration of hepatic and muscle glycogen concentrations to near normalcy. We suggest that the OA-induced increase in glycogen levels was due to OA's insulin mimetic effects because HK and GK are both insulin-dependent and insulin-sensitive enzymes.Citation2 This point is underscored by the observation that OA significantly increased muscle glycogen concentration of STZ-induced diabetic rats with low insulin levels.Citation19–24 Glycogen synthesis in skeletal tissues is dependent on insulin that stimulates translocation of the GLUT-4 to the cell membrane to mediate glucose uptake.Citation25–29 Therefore, it is evident that previously reported improvement in the glycemic state of STZ-induced diabetic rats by OACitation11 can be attributed to the modulation of activities of glycogenic enzymes perhaps due to the triterpene possessing insulin mimetic effects.
Interestingly, combined OA and insulin had an even greater effect on restoring muscle glycogen concentrations in STZ-induced diabetic rats, which is suggestive of a synergistic effect of the two compounds. However, this treatment did not significantly alter the activities of muscle HK and GK in these rats. Furthermore, this treatment did not show any additive effects of glycogen levels in the nondiabetic rats perhaps due to the masking effects of endogenous insulin.
In view of the pronounced effect of OA and insulin on glycogen, especially hepatic glycogen, in STZ-induced diabetic rats, it seems odd that this treatment had no effect on HK or GK activity, and yet both were able to increase activity in STZ rats. The results from this study cannot explain this anomaly. Perhaps glycogen synthesis from other precursors such as amino acids (alanine or glutamine), fructose, and lactate whose pathway is independent of these enzymesCitation30 can partly explain this discrepancy. Indeed, the effects of fructose on glycogen synthase, a key glycogenic enzyme, are independent of GK activation.Citation31 Furthermore, triterpenoids have over the years been found to stimulate glycogen synthesis not only by increasing the activities of HK, GK, GS,Citation32 but also by inhibiting the activity of glycogen phosphorylase thereby decreasing hepatic glucose production.Citation33 Reports indicate that S. aromaticum crude extract depresses the activities of hepatic gluconeogenic enzymes, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase,Citation34 thereby inhibiting hepatic glucose production. The restoration of this principal glucose utilization pathway by OA though would constitute a novel therapeutic strategy for diabetes treatment as reported for some antidiabetic drugs that lower blood glucose by activating glycogenic enzymes.Citation35
Taken together with previous observations, our data suggest that OA administration restores the activity of key glycogenic enzymes in the liver and skeletal muscle of STZ-induced diabetic rats to enhance glycogen synthesis to improve the glycemic status. Further studies are needed to establish the effects of the triterpene on glycogen synthase.
ACKNOWLEDGMENT
The authors are grateful to Professor FAO Shode and Mr. OO Oyedeji, School of Chemistry, University of KwaZulu-Natal, for assistance in phytochemical studies and the Biomedical Research Unit for assistance with study animals.
Declaration of interest:
The authors declare that there is no interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Murphy ED, Anderson JW. Tissue glycolytic and gluconeogenic enzyme activities in mildly and moderately diabetic rats. Influence of tolbutamide administration. Endocrinology. 1974;94:27–34.
- Hornbrook KR. Synthesis of liver glycogen in starved alloxan diabetic rats. Diabetes. 1970;19:916–923.
- Whitton PD, Hems DA. Glycogen synthesis in the perfused liver of streptozotocin-diabetic rats. Biochem J. 1975;150:153–165.
- Postic C, Shiota M, Magnuson MA. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog Horm Res. 2001;56:195–217.
- Iynedjian PB. Mammalian glucokinase and its gene. Biochem J. 1993;293:1–13.
- Wilson JE. Distinguishing the type I and type II isozymes of hexokinase: The need for a reexamination of past practice. Diabetes. 1998;47:1544–1548.
- Weber G, Lea MA, Fisher EA, Stamm NB. Regulatory pattern of liver carbohydrate metabolizing enzymes insulin as an inducer of key glycolytic enzymes. Enzymology Clin. 1966;7:11–24.
- Gershell L. Type 2 diabetes market. Nat Rev Drug Discov. 2005;4:367–368.
- Taylor R, Agius L. The biochemistry of diabetes. Biochem J. 1988;250(3):625–640.
- Musabayane CT, Mahlalela N, Shode FO, Ojewole JAO. Effects of Syzygium cordatum (Hochst.)[Myrtaceae] leaf extract on plasma glucose and hepatic glycogen in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;97:485–490.
- Musabayane CT, Tufts MA, Mapanga RF. Synergistic anti-hyperglycemic effects between plant derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Ren Fail. 2010;32(7):832–839.
- Jang SM, Kim MJ, Choi MS, Kwon EY, Lee MK. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism. 2009;59(4):512–519.
- Mapanga RF, Tufts MA, Shode FO, Musabayane CT. Renal effects of plant derived oleanolic acid in streptozotocin-induced diabetic rats. Ren Fail. 2009;31(6):481–491.
- Ong KC, Khoo HE. Effects of myricetin on glycaemia and glycogen metabolism in diabetic rats. Life Sci. 2000;67:1695–1705.
- Gondwe M, Kamadyaapa DR, Tufts MA, Chuturgoon AA, Ojewole JAO, Musabayane CT. Effects of Persea americana Mill (Lauraceae)[“Avocado”] ethanolic extract on blood glucose and kidney function in streptozotocin-induced diabetic rats and on kidney cell lines of the proximal (LLC-PK1) and distal tubules (MDBK). Methods Find Exp Clin Pharmacol. 2008;30(1):25–35.
- Goward CR, Hartwell R, Atkinson T, Scawen MD. The purification and characterization of glucokinase from the thermophile Bacillus stearothermophilus. J Biochem. 1986;237:415–420.
- Salas J, Salas M, Vinuela E, Sols A. Glukokinase of rabbit liver. J Biol Chem. 1965;240:1014–1018.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagents. J Biol Chem. 1951;193:265–275.
- Dent P, Lavoinne A, Nakienly S, Watt P, Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990;348 (6299):302–308.
- Flodstrom M, Tyrberg B, Eizirik DL, Sandler S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999;48:706–713.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6): 537–546.
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercitin in streptozotocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C(3):357–364.
- Vats V, Yadav SP, Grover JK. Effect of T. foenumgraecum on glycogen content of tissues and the key enzymes of carbohydrate metabolism. J Ethnopharmacol. 2003;85:237–242.
- Denis DM, Miri A, Bielicki G, Mignon M, Renou JP, Grizard J. Insulin-dependent glycogen synthesis is delayed in onset in the skeletal muscle of food-deprived rats. J Nutr Biochem. 2005;16:150–154.
- Barnard RJ, Youngren JF. Regulation of glucose transport in skeletal muscle. FASEB J. 1992;6:3238–3244.
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001; 414(6865):799–806.
- Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycaemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod. 2003;66:788–792.
- Jensen J, Jebens E, Brennesvik EO, Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am J Physiol Endocrinol Metab. 2006;290:E154–E162.
- Wiernsperger NF. Is non-insulin dependent glucose uptake a therapeutic alternative? Part 1: Physiology, mechanisms and role of non-insulin dependent glucose uptake in type 2 diabetes. Diabetes Metabolism. 2005;31:415–426.
- Newgard CB, Hirsch LJ, Foster DW, McGarry JD. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. J Biol Chem. 1983;258:8046–8052.
- Gergely P, Toth B, Farkas L, Bot G. Effects of fructose 1‐phosphate on the activation of liver glycogen synthase. J Biochem. 1985;232:133–137.
- Kumari K, Mathew BC, Augusti KT. Anti diabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Ind J Biochem Biophys. 1995;32:49–54.
- Chen J, Liu J, Zhang L, Pentacyclic triterpenes. Part 3: Synthesis and biological evaluation of oleanolic acid derivatives as novel inhibitors of glycogen phosphorylase. Bioorg Med Chem Lett. 2006;16(11):2915–2919.
- Prasad RC, Herzog B, Boone B, Sims L, Waltner-Law M. An extract of Syzygium aromaticum represses genes encoding gluconeogenic enzymes. J Ethnopharmacol. 2005;96:295–301.
- Gupta D, Raju J, Prakash J, Baquer NZ. Change in the lipid profile, lipogenic and related enzymes in the livers of experimental diabetic rats: Effects of insulin and vanadate. Diabetes Res Clin Pract. 1999;46:1–7.